Robo2 acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons
Figures
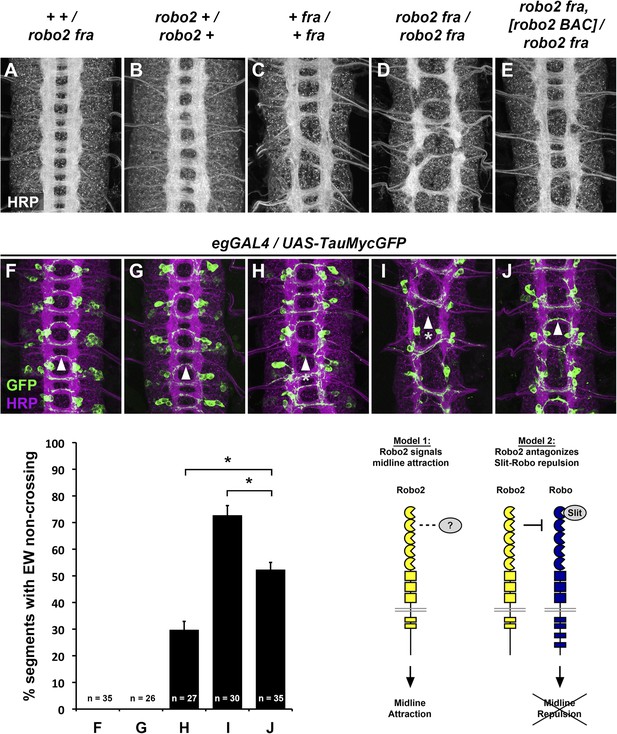
Robo2 commissural guidance defects are rescued by a Robo2 BAC transgene.
(A–E) Stage 17 Drosophila embryos of the indicated genotypes stained with anti-HRP antibodies to label all CNS axons. (F–J) Stage 15–16 embryos of the indicated genotypes carrying eg-GAL4 and UAS-TauMycGFP transgenes, stained with anti-HRP and anti-GFP antibodies. Anti-GFP labels cell bodies and axons of the eagle neurons (EG and EW) in these embryos. (A and F) Embryos heterozygous for both frazzled (fra) and robo2 display a wild-type arrangement of longitudinal and commissural axon pathways, and axons of the EW neurons cross the midline in the posterior commissure in 100% of segments (arrowhead). (B and G) robo2 mutants (robo2123/robo233) display a mildly disorganized axon scaffold, but no detectable defects in EW crossing. (C and H): fra mutants (fra3/fra4) display thinning commissures indicative of decreased midline crossing, and the EW axons fail to cross the midline in 30% of abdominal segments (arrowhead with asterisk). (D and I) Simultaneous removal of robo2 and fra (robo2123, fra3/robo2135, fra4) strongly enhances the midline crossing defects seen in fra single mutants. (E and J) Midline crossing is partially restored in robo2, fra double mutants carrying one copy of an 83.9-kb robo2 BAC transgene. The overall organization of the axon scaffold approaches that seen in fra single mutants, and EW axon crossing defects are significantly rescued, although not completely restored to the level seen in fra single mutants. Histogram quantifies EW midline crossing defects in the genotypes shown in (F–J). Error bars represent s.e.m. n, number of embryos scored for each genotype (*p<0.0001). Bottom right: Two possible models for how Robo2 might promote midline crossing of commissural axons. Left, Robo2 may act as a midline attractive receptor to promote midline crossing in response to an unidentified ligand, analogous to Fra's role as an attractive Netrin receptor. Right, Robo2 may antagonize canonical Slit-Robo1 repulsive signaling to down-regulate midline repulsion and thus allow Robo1-expressing axons to cross the midline.
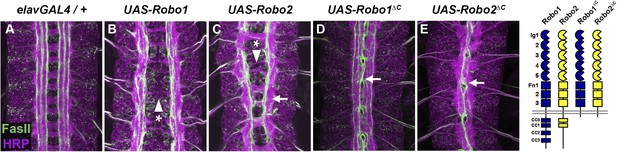
Robo2 can promote midline crossing independent of its cytoplasmic domain.
(A–E) Stage 17 embryos carrying elav-GAL4 and the indicated UAS-Robo transgenes, stained with anti-HRP (magenta) and the longitudinal pathway marker anti-FasciclinII (FasII; green). (A) Embryos carrying elav-GAL4 alone exhibit a wild-type arrangement of axon pathways, including distinct anterior and posterior commissures and three FasII-positive longitudinal pathways that do not cross the midline. (B) In elav-GAL4/UAS-Robo1 embryos, commissure formation is strongly impaired, and no ectopic midline crossing of FasII-positive axons is observed. (C) Mis-expression of Robo2 with elav-GAL4 produces a biphasic phenotype, where some segments appear nearly commissureless (arrowhead with asterisk) while others exhibit ectopic crossing reminiscent of robo1 mutants (arrow). See Figure 5 for quantification of ectopic crossing in elav-GAL4/UAS-Robo2 embryos. (D and E) Mis-expression of truncated forms of Robo1 (Robo1∆C) or Robo2 (Robo2∆C) with elav-GAL4 induces ectopic crossing in 100% of segments, although the Robo2∆C mis-expression phenotype is qualitatively more severe than Robo1∆C. In elav-GAL4/UAS-Robo1∆C embryos (D) only the medial FasII pathway crosses the midline and the axon scaffold overall exhibits a robo1-like appearance, while in elav-GAL4/UAS-Robo2∆C embryos (E) all three FasII-positive pathways collapse at the midline in nearly every segment and the axon scaffold appears slit-like. All UAS-Robo transgenes shown here were inserted into the same genomic location (86FB) to ensure equivalent expression levels.
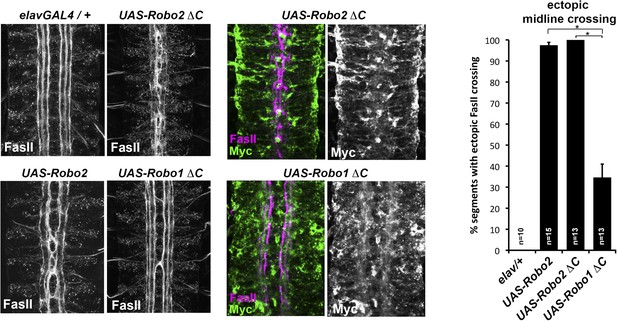
Comparison of Robo1∆C and Robo2∆C gain of function activities.
Since the effects of expressing ∆C transgenes in the 86Fb insertion site are too potent to allow quantitative comparison, we used traditional UAS insertion lines that are expressed at lower and comparable levels (right panels, anti-Myc is shown in green and anti-FasII in magenta) to compare activities of Robo1∆C and Robo2∆C. In embryos expressing only an elav-GAL4 transgene (top left), FasII axons appear wild-type and remain ipsilateral. Mis-expression of Robo2 leads to a high level of ectopic crossing. Robo2∆C expression results in a much greater degree of ectopic midline crossing than does Robo1∆C. Segments with ectopic midline crossing of FasII axons are quantified on the right. Significance was assessed by multiple comparisons using the Student's t-test and a Bonferonni correction (*p < 0.001). Error bars represent s.e.m. n, number of embryos scored for each genotype.
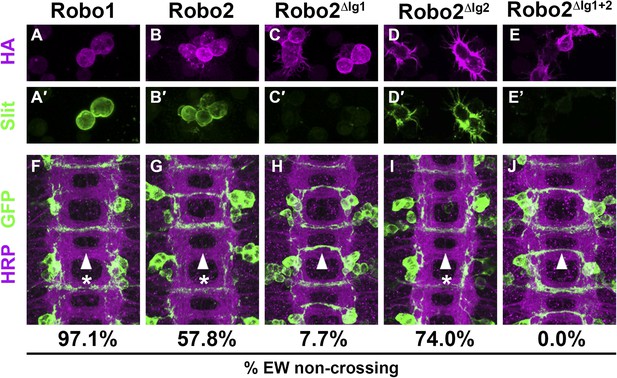
Slit binding and Robo gain of function.
(A–E) Slit-conditioned media was collected and used to treat cells expressing the indicated HA-tagged receptors. Receptor expression is shown with anti-HA in the top panels (magenta) and anti-Slit staining is shown in the bottom panels (green, A′–E′). Robo1 (A), Robo2 (B), and Robo2∆Ig2 (D) bind efficiently to Slit, while little to no binding is detected in cells expressing Robo2∆Ig1 (C) or Robo2∆Ig1+2 (E). (F–J) Stage 16 embryos expressing the indicated transgene in the Eg commissural interneurons. HRP labels the axon scaffold (magenta) and anti-GFP labels the Eg neurons. The percentages under each panel indicate the percentage of EW axons that fail to cross the midline in each condition. Expression of Robo1 (F), Robo2 (G), and Robo2∆Ig2 (I) all lead to strong disruption of midline crossing, while expression of Robo2∆Ig1 (H), and Robo2∆Ig1+2 (J) result in little to no crossing defects.
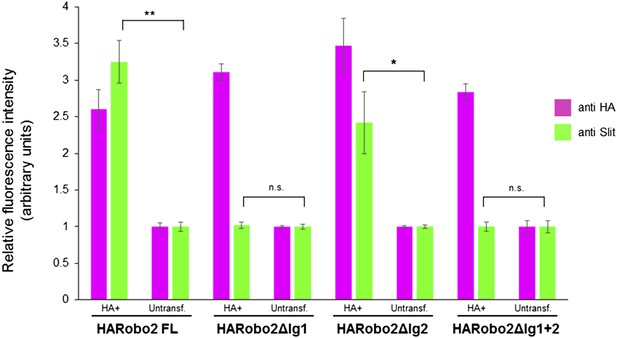
Quantification of relative fluorescence intensity of HA and Slit antibody staining in S2R+ cells transfected with HA-tagged Robo2 proteins, and treated with Slit-conditioned media.
Fluorescence intensities are normalized to the average pixel intensity of untransfected cells from the same slide. The values from three images per Robo2 variant were averaged; error bars represent the standard error of the mean. * denotes p < 0.03 and ** denotes p < 0.01 from a Student's t-test.
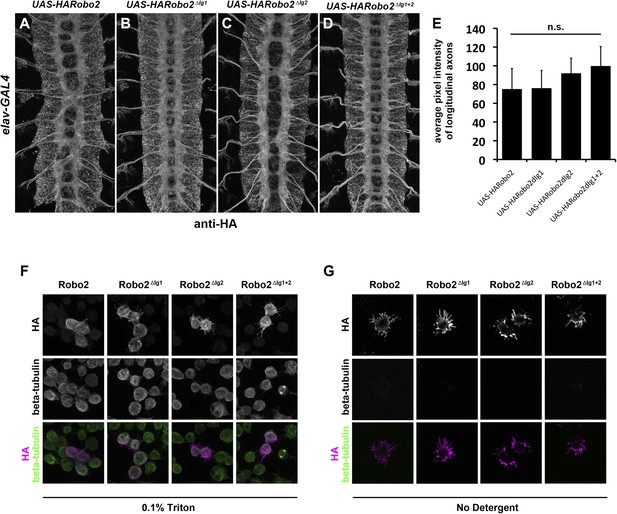
Robo2 transgenes are localized to axons and expressed at equivalent levels in vivo and are present at the surface of S2R+ cells in vitro.
(A–E) Embryos carrying elav-GAL4 and the indicated UAS-Robo2 transgenes were stained with anti-HA antibodies and imaged via confocal microscopy. Staining and imaging conditions were identical for all samples. (A–D) Representative images of embryos expressing each transgene and stained with anti-HA. All of the Robo2 variants are localized to axons when expressed pan-neurally with elav-GAL4. (E) Quantification of pixel intensity for each transgenic line. Confocal max projections through the entire neuropile were collected for three stage 16 embryos for each line, and average pixel intensity was measured across five 25-pixel regions within the longitudinal axon pathways for each embryo. Bar graph shows average pixel intensity across the three embryos for each line. Error bars indicate standard deviation. Average pixel intensity values were not significantly different for any of the four transgenic lines by Student's t-test (p > 0.2 for all comparisons). (F) S2R+ cells transfected with the indicated Robo2 constructs were permeabilized and stained with anti-HA and anti-tubulin antibodies. No differences were observed in the localization or expression of the different HA-Robo2 variants. Staining and imaging conditions were identical for all samples. (G) S2R+ cells transfected with the indicated Robo2 constructs were incubated with anti-HA and anti-tubulin antibodies at 4°C for 30 min, in the absence of detergent. All Robo2 proteins were robustly detected at the cell surface by this method, with no noticeable differences in localization or staining intensity; no tubulin signal was detected, confirming that cells were not permeabilized. Staining and imaging conditions were identical for all samples.
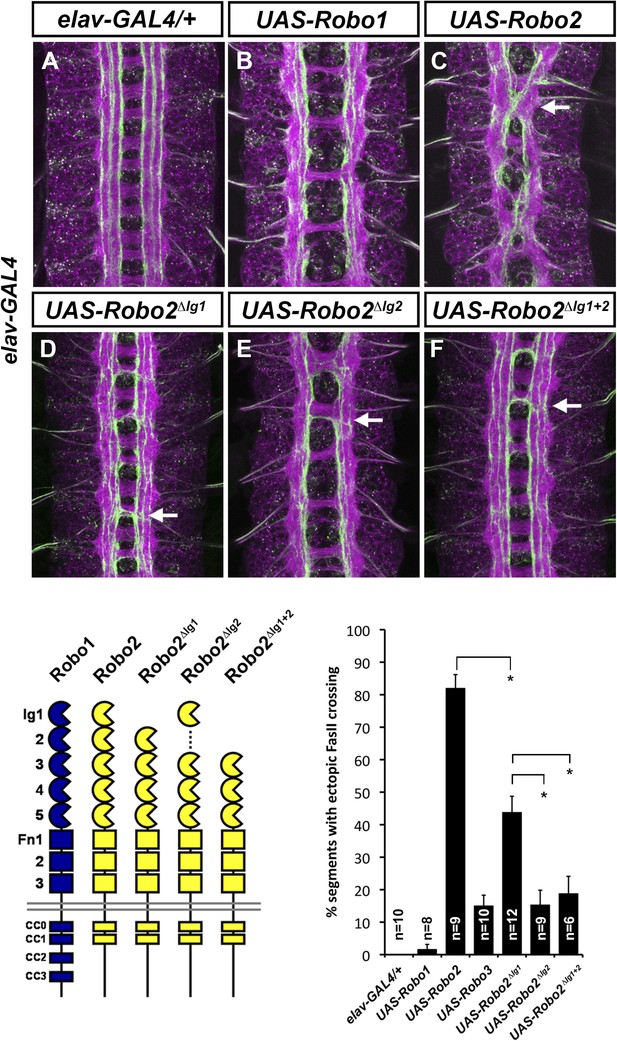
Robo2's pro-crossing activity depends on its Ig2 domain.
(A–F) Stage 17 embryos carrying elav-GAL4 and the indicated UAS-Robo transgenes, stained with anti-HRP and anti-FasII. (A) Embryos carrying elav-GAL4 alone exhibit a wild-type arrangement of axon pathways, including three FasII-positive longitudinal pathways that do not cross the midline. (B) Robo1 does not promote midline crossing of FasII-positive axons when misexpressed in all neurons with elav-GAL4. (C) Misexpression of full-length Robo2 induces ectopic midline crossing in over 80% of segments (arrow). (D) Deleting the Ig1 domain (Robo2∆Ig1) disrupts Slit binding but does not completely prevent Robo2 from promoting midline crossing. (E and F) Robo2 receptors lacking the Ig2 domain (Robo2∆Ig2) or both the Ig1 and Ig2 domains (Robo2∆Ig1+2) are unable to promote ectopic midline crossing above background levels (both are comparable to Robo3; see histogram). Schematics show domain composition of receptors shown in (A–F). All UAS-Robo transgenes shown here were inserted into the same genomic location (86FB) to ensure equivalent expression levels. Histogram quantifies ectopic midline crossing in the indicated genotypes. Significance was assessed by multiple comparisons using the Student's t-test and a Bonferonni correction (*p < 0.01). Error bars represent s.e.m. n, number of embryos scored for each genotype.
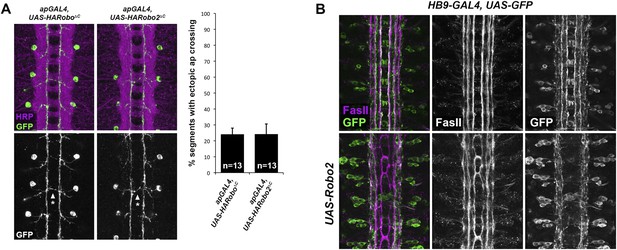
Robo2 acts cell non-autonomously to promote midline crossing in ipsilateral neurons.
(A) Stage 17 embryos stained with anti-HRP (magenta) and anti-GFP (green) antibodies. Anti-GFP labels the apterous (ap) cell bodies and axons, which normally project ipsilaterally. Mis-expression of Robo2ΔC in ap neurons results in a mild ectopic crossing phenotype, which is similar to the effect of Robo1ΔC (arrowheads with asterisks). Segments with ectopic crossing of ap axons are quantified in the histogram. (B) Stage 17 embryos stained with anti-FasII (magenta) and anti-GFP (green) antibodies. Anti-GFP labels the axons of hb9-GAL4 expressing cells. Mis-expression of Robo2 with hb9-GAL4 results in a lateral shift of hb9-Gal4+ axons, and causes FasII+ axons that do not express hb9-GAL4 to ectopically cross the midline.
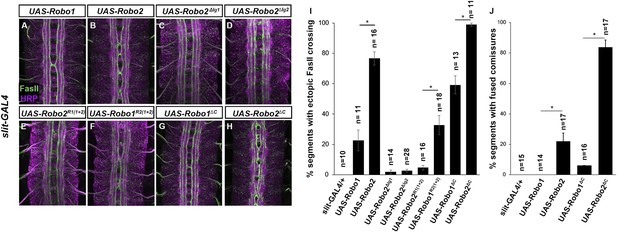
Robo2 can promote crossing non cell-autonomously.
(A–D) Stage 17 embryos stained with anti-HRP (magenta) and anti-FasII (green). (A and B) Mis-expression of Robo1 (A) in midline cells using slit-GAL4 results in a mild ectopic crossing phenotype. In contrast, mis-expression of Robo2 (B) produces a much stronger effect, as indicated by quantification of ectopic FasII crossing in the histogram (I). (C and D) Mis-expression of either Robo2∆Ig1 (C) or Robo2∆Ig2 (D) with slit-GAL4 does not produce ectopic crossing of FasII axons. (E and F) Consistent with requirement of Robo2's first two IG domains, the chimeric protein Robo1R2IG(1+2) produces an ectopic crossing phenotype (F), whereas Robo2R1(IG1+2) has no effect (E). (G and H) Mis-expression of Robo2∆C with slit-GAL4 also results in severe ectopic crossing defects (H) that are much stronger than those observed with Robo1∆C (G), as indicated by quantification of ectopic FasII crossing (I) and fused commissures observed in anti-HRP stained embryos (J). All UAS-Robo transgenes were inserted into the same genomic location (86FB). Significance was assessed by multiple comparisons using the Student's t-test and a Bonferonni correction (*p < 0.001). Error bars represent s.e.m. n, number of embryos scored for each genotype.
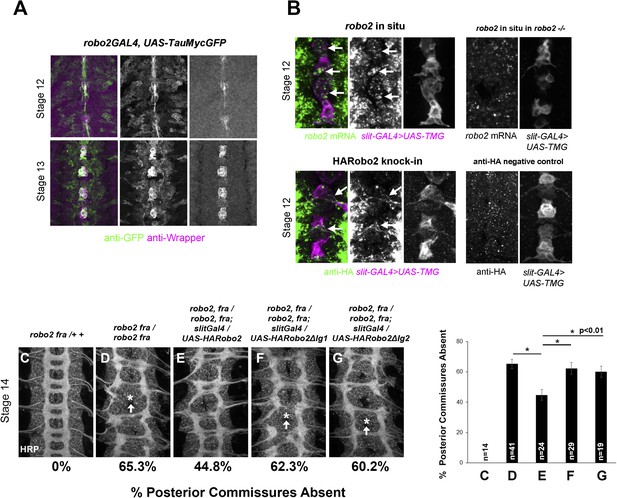
robo2 is expressed in midline cells during commissural axon path finding, and over-expressing robo2 with slit-GAL4 restores midline crossing in robo2, fra double mutants.
(A) A robo2-GAL4 enhancer trap that recapitulates robo2's endogenous expression pattern drives UAS-TauMycGFP expression (green) in midline cells at stages 12–13, when many commissural axons cross the midline. Midline glia are labeled by an anti-wrapper antibody (magenta). (B) Top: Fluorescent in situ for robo2 mRNA (green). robo2 is transiently expressed in midline glia and neurons (magenta) during stage 12 (arrows). This in situ signal is not observed in robo2 mutant embryos confirming the specificity of the mRNA detection (right). (B) Bottom: Robo2 protein is expressed in midline cells during the stages of commissural axon path finding, as shown by the expression pattern of a HA-tagged robo2 cDNA knock-in allele (robo2HArobo2). Stage 12 embryos carrying robo2HArobo2, slit-GAL4, and UAS-TauMycGFP show HARobo2 expression in slitGAL4-expressing cells (arrows), whereas this signal is not detected in control embryos (right). (C–G) Stage 14 embryos of the indicated genotypes stained with anti-HRP antibodies to label all CNS axons. The absence of PC was scored in abdominal segments A1–A8 (arrows indicate examples of missing commissures). The PC defects of robo2, fra double mutants (D) are significantly rescued by over-expressing UAS-Robo2 with slit-GAL4 (E), whereas over-expression of UAS-Robo2ΔIg1 (F) or UAS-Robo2ΔIg2 (G) has no effect. Embryos were scored blind to genotype. Significance was assessed by one-way ANOVA followed by multiple comparisons using the Student's t-test and a Bonferonni correction (*p < 0.01). Error bars represent s.e.m. n, number of embryos scored for each genotype.
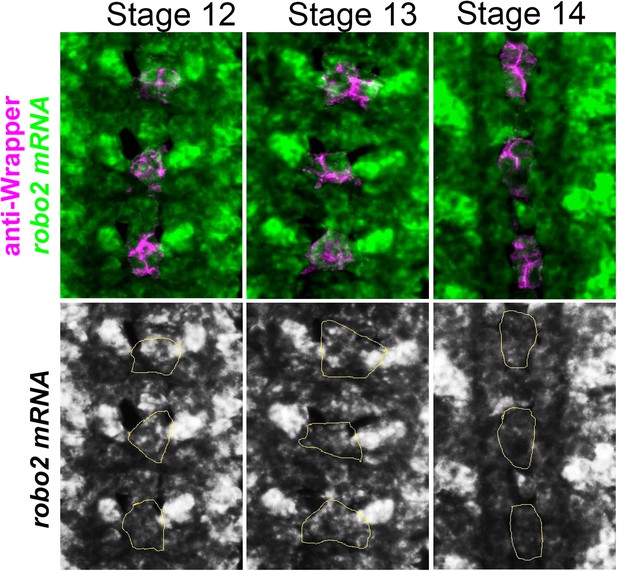
robo2 mRNA is transiently expressed in midline cells.
Fluorescent in situ for robo2 mRNA (green). robo2 is transiently expressed in midline glia (magenta) during Stages 12 and 13 but is no longer detected there by Stage 14. Midline glia are labeled with an antibody to Wrapper.
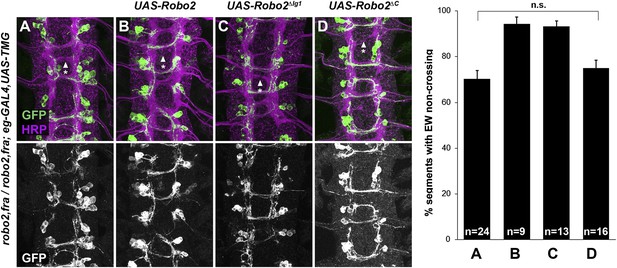
Robo2 cannot rescue midline crossing cell autonomously.
(A–D) Stage 16 embryos of the indicated genotypes stained with anti-HRP (magenta) and anti-GFP (green) antibodies. Anti-GFP labels the EG and EW cell bodies and axons. EW crossing defects in robo2, fra double mutants (A) are not rescued by eg-GAL4 mediated over-expression of UAS-Robo2 (B), UAS-Robo2ΔIG1 (C), or UAS-Robo2ΔC (D), suggesting that Robo2 cannot act cell autonomously to promote midline crossing. Segments with non-crossing EW axons are indicated by arrowheads with asterisks. Significance was assessed by multiple comparisons using the Student's t-test and a Bonferonni correction. No significant differences between any of the genotypes were observed (p > 0.3). Error bars represent s.e.m. n, number of embryos scored for each genotype.
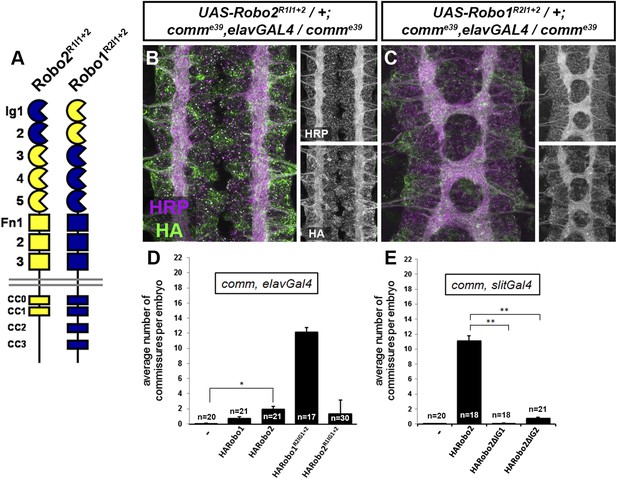
Robo2 receptors that promote midline crossing suppress comm mutants.
(A) Schematic diagram of the two chimeric receptors shown in (B and C). Robo1 sequences are depicted in blue and Robo2 sequences are depicted in yellow. (B and C) Stage 16 embryos of the indicated genotype stained with anti-HRP to visualize CNS axons and anti-HA to visualize the epitope tagged chimeric receptor. Single channel images of HRP and HA are presented to the right of the color panels. Expression of the HA-Robo2R1Ig1−2 chimeric receptor in a comm mutant background (B) does not restore commissure formation, while expression of the reciprocal HA-Robo1R2Ig1+2 chimeric receptor (C) strongly suppresses the comm mutant phenotype. (D and E) Quantification of the average number of commissures per embryo in comm mutants expressing the indicated HA-tagged receptor transgenes in either all neurons using elav-GAL4 (D) or in midline cells using slit-GAL4 (E). Significance was assessed by one-way ANOVA followed by multiple comparisons using the Student's t-test and a Bonferonni correction (*p < 0.0001) (**p < 1.0 e−10). Error bars represent s.e.m. n, number of embryos scored for each genotype.
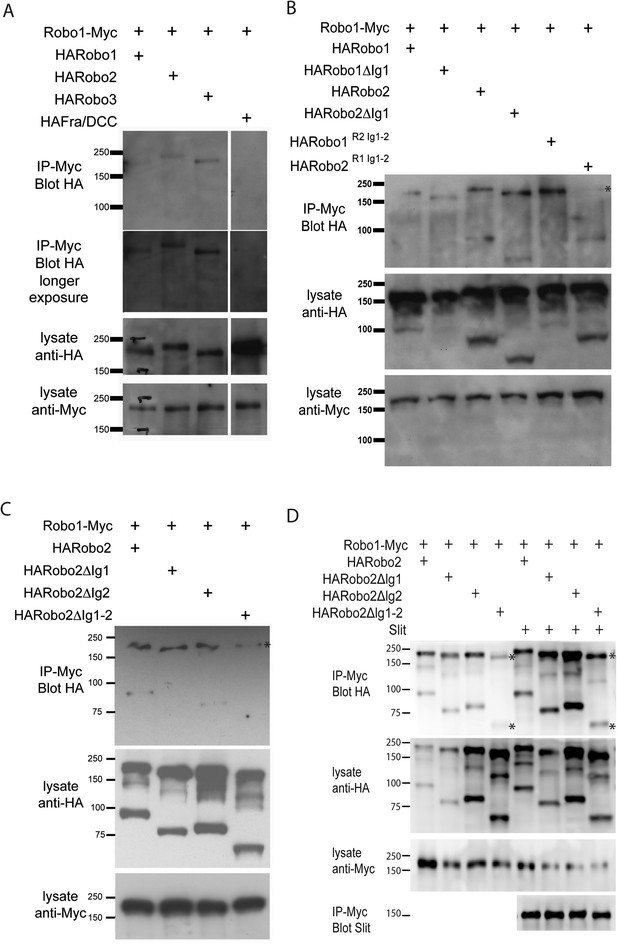
Robo2 binds to the Robo1 receptor in vitro and in vivo.
(A–C) Protein extracts from embryos expressing Robo1-Myc and various HA-tagged receptors in all neurons were immunoprecipitated with anti-Myc antibodies and analyzed by western blot. Immunoprecipitates were probed with anti-HA (top blots) and total lysates were compared for HA expression and Myc expression to ensure that equal inputs were analyzed. Representative western blots from multiple experiments are shown. (A) Robo1-Myc binds to HARobo1, HARobo2, and HARobo3 but not to a HA-tagged Fra receptor (two exposures are shown). Total lysate blots reveal comparable loading with the exception of the Fra negative control in which there is substantially more HA-tagged receptor. (B) Robo1-Myc binds efficiently to HARobo2, HARobo2∆Ig1, and the HARobo1Robo2(IG1-2) chimera but not to the reciprocal chimera that has Ig1 and Ig2 domains from Robo1 (asterisk). (C) Deletion of either Robo2 Ig1 or Ig2 alone does not substantially affect Robo1 binding, while deleting both domains results in reduced binding (asterisk). See Figure 11—figure supplement 1 for an additional example. (D) Cell lysates of S2R+ cells separately transfected for Robo1-Myc or HA-tagged Robo2 variants were mixed, immunoprecipitated with anti-Myc, and analyzed by western blot. In this assay, Robo1-Myc binds efficiently to HARobo2, HARobo2∆Ig1, and HARobo2∆Ig2, and less well to HARobo2∆Ig1+2 (asterisks). In lanes 1–4, cells were untreated; in lanes 5–8, cells were treated with Slit-conditioned media before lysing. We note that in addition to detection of the predicted full-length Robo2 receptor with anti-HA, we also routinely detect a smaller ∼80 kD fragment that corresponds to an extracellular domain cleavage product. The size of this fragment is shifted to predictably smaller sizes when Ig1, Ig2, or both Ig1 and Ig2 are deleted. We do not currently know, whether this cleavage event is required for Robo2 function in any context, since we have not been able to generate an uncleavable version of the receptor.
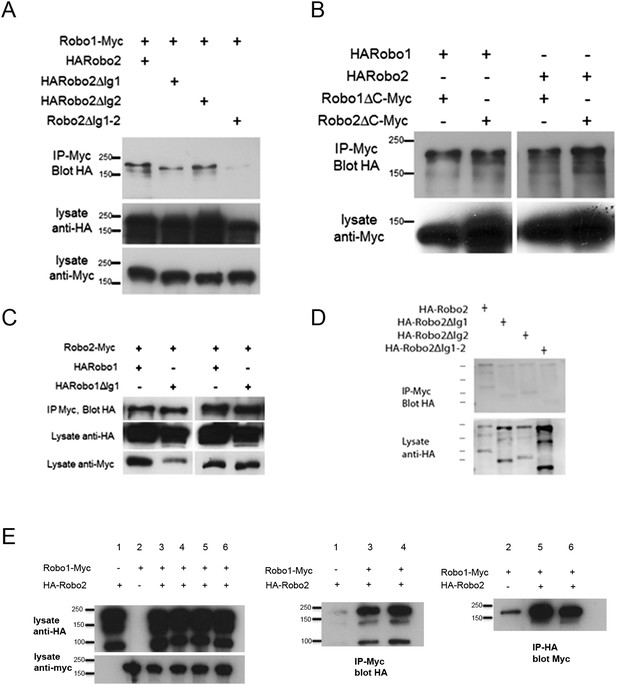
Robo2 binding to Robo1 does not depend on its cytoplasmic domain or on Robo1's Ig1 domain.
(A–C) Protein extracts from embryos expressing Robo1-Myc and various HA-tagged receptors in all neurons were immunoprecipitated with anti-Myc antibodies and analyzed by western blot. Immunoprecipitates were probed with anti-HA (top blot) and total lysates were compared for HA expression and Myc expression to ensure that equal inputs were analyzed. Representative western blots from multiple experiments are shown. (A) Deletion of either Robo2 Ig1 or Ig2 alone does not substantially affect Robo1 binding, while deleting both domains results in reduced binding. (B) Extracts from embryos co-expressing either HARobo1 or HARobo2, and either Robo1∆C-Myc or Robo2∆CMyc were analyzed for interactions. Both of the C-terminal truncation receptor variants can efficiently pull down both HARobo1 and HARobo2 indicating that binding is independent of the cytoplasmic domain. (C) Similar experiments to those described above and in the legend to the main Figure 11 indicate that Robo2 does not bind to the Slit-binding Ig1 region of Robo1. (D) Lysates of S2R+ cells expressing HA-tagged Robo2 variants were mixed with lysates of untransfected cells, and immunoprecipitated with anti-Myc as a negative control for the experiment in Figure 11D. Very little Robo2 protein was detected in the immunoprecipitates. (E) S2R+ cells were co-transfected with Robo1-Myc and HA-Robo2 and immunoprecipitated with anti-Myc (middle panel) or anti-HA (right panel). A strong interaction was detected between Robo1 and Robo2 when the pull-down was performed in either direction.
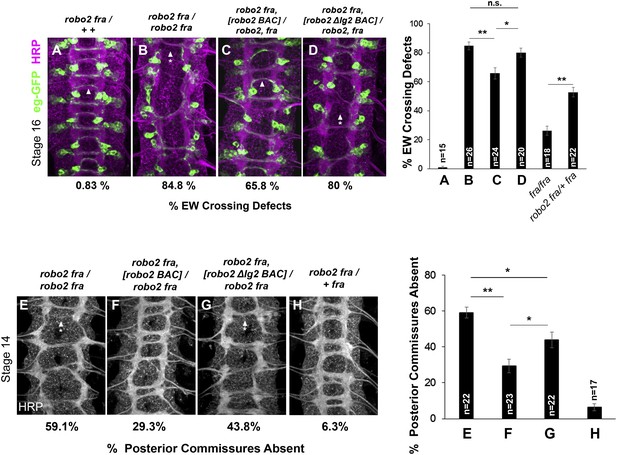
Robo2's endogenous activity in promoting midline crossing depends on Ig2.
(A–D) Stage 16 embryos stained with anti-HRP (magenta) and anti-GFP (green) antibodies. Anti-GFP labels the EG and EW cell bodies and axons. Arrowheads indicate EW axons that have crossed the midline and arrowheads with asterisks indicate non-crossing EW axons. (A) Almost all EW axons cross the midline in robo2, fra/+, + double heterozygotes. (B) EW crossing defects are observed in 85% of segments in robo2, fra double mutants. (C–D) The FL Robo2 cDNA BAC transgene (C) significantly rescues EW crossing, to 66% of segments with defects (Student's t-test, **p < 0.001) whereas the Robo2ΔIG2 transgene (D) does not significantly rescue. Right: Removing one copy of robo2 significantly enhances midline crossing defects in fra mutants. (E–H) Stage 14 embryos of the indicated genotypes stained with anti-HRP. Posterior commissures were scored in abdominal segments A1–A8. Missing posterior commissures are indicated by arrowheads with asterisks. (E–G) The posterior commissure defects of robo2, fra double mutants are significantly rescued by a full-length (FL) Robo2 cDNA BAC transgene (Student's t-test, **p < 0.001) (F), as well as by a Robo2ΔIG2 BAC (*p < 0.0167) (G). The Robo2ΔIG2 BAC does not rescue as well as FL Robo2 (*p < 0.0167). All embryos were scored blind to genotype. Significance was assessed by one-way ANOVA followed by multiple comparisons using the Student's t-test and a Bonferonni correction. Error bars represent s.e.m. n, number of embryos scored for each genotype.
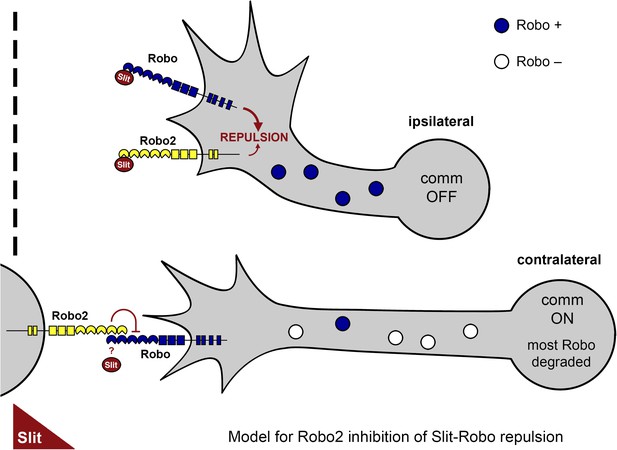
A model of Robo2's pro-crossing function.
In contralateral neurons, the endosomal sorting receptor Comm is expressed, and it prevents the majority of Robo1 from reaching the growth cone surface. We propose that Robo2 acts non-autonomously in midline cells to bind to and inhibit the low level of Robo1 that escapes Comm-dependent sorting. This mechanism is revealed in contexts where axon attraction to the midline is limited. In ipsilateral neurons, Comm is not expressed, and Robo2 works together with Robo1 to mediate repulsion from the midline in response to Slit.





