ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure
Figures
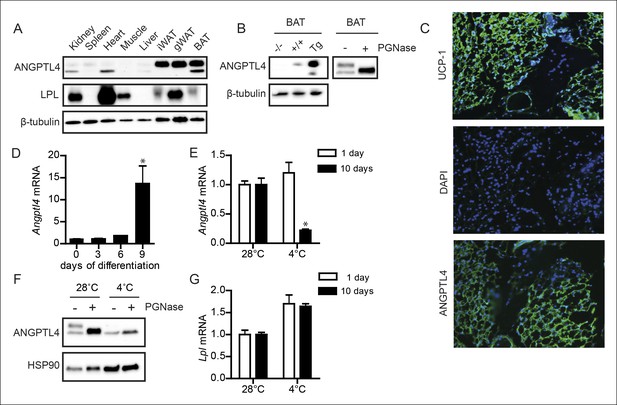
ANGPTL4 expression in BAT is down-regulated upon sustained cold exposure.
(A) Immunoblot for mouse ANGPTL4 and mouse LPL in lysates of kidney, spleen, heart, muscle, gonodal WAT, inguinal WAT and BAT of a C57BL/6J wild-type mouse. (B) Validation of anti-mANGPTL4 antibody in BAT lysates of Angptl4-/-, wild-type and Angptl4-Tg mice (left panel). Detection of glycosylated and non-glycosylated mANGPTL4 following treatment of BAT homogenate of a wild-type mouse with PGNase (right panel). (C) Immunofluorescent staining of UCP1 (upper panel; UCP1 = green, DAPI = blue), DAPI only (middle panel) and hANGPTL4 (lower panel; hANGPTL4 = green, DAPI = blue) in frozen sections (5 μm) of human BAT. (D) Angptl4 mRNA in T37i cells after 0, 3, 6 or 9 days of differentiation. (E) Angptl4 mRNA in BAT lysates of wild-type mice exposed to 4°C or 28°C for 1 or 10 days. (F) Immunoblot for ANGPTL4 in BAT homogenates of wild-type mice exposed to 4°C or 28°C for 10 days, following treatment with or without PGNase. (G) Lpl mRNA in BAT lysates of wild-type mice exposed to 4°C or 28°C for 1 or 10 days. * Statistically significant compared to control wells or compared to mice exposed to 28°C according to Student’s t-test (p<0.05). Error bars represent ± SEM. n = 8–10 mice per group.
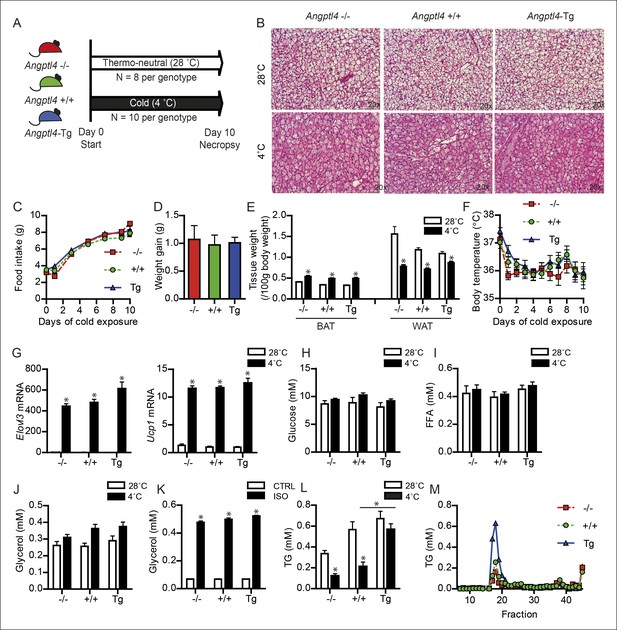
Down-regulation of ANGPTL4 in BAT upon sustained cold exposure affects plasma TG levels.
(A) Schematic representation of cold exposure experiment with Angptl4-/-, wild-type and Angptl4-Tg mice. (B) Haematoxylin & Eosin staining on BAT sections (5 μm) of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (C) Food intake of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C for 10 days. (D) Weight gain of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C during 10 days. (E) BAT and WAT tissue weights and (F) body temperature of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (G) Elovl3 and Ucp1 mRNA expression levels of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (H) Plasma glucose, (I) plasma free fatty acids, and (J) plasma glycerol levels of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (K) Glycerol levels in medium of differentiated primary white adipocytes from Angptl4-/-, wild-type and Angptl4-Tg mice, serum-starved and treated with 10 μM isoproterenol for 3 hr. (J) plasma TG levels of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (K) Fast protein liquid chromatography (FPLC) on pooled plasma samples of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C for 10 days, followed by analysis of TG levels in all fractions. *Statistically significant compared to mice of equal genotype at 28°C or between groups as indicated by bars according to two-way ANOVA followed by a post-hoc Tukey HSD test (p<0.05). Error bars represent ± SEM. n = 8–10 mice per group.
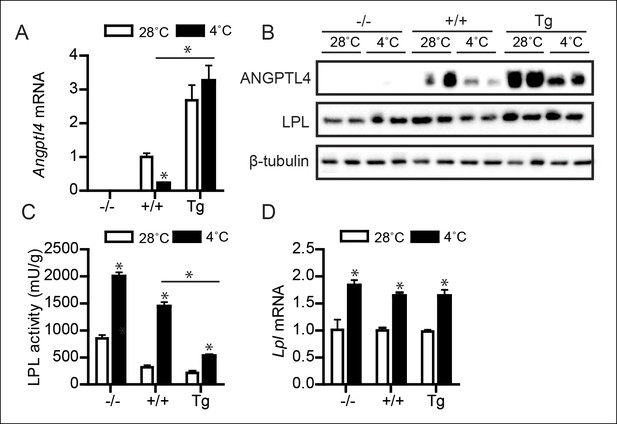
Down-regulation of ANGPTL4 in BAT upon sustained cold exposure promotes an increase in BAT LPL activity.
(A) Angptl4 mRNA in BAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (B) Immunoblot for ANGPTL4 and LPL in BAT homogenates from Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (C) Total LPL activity and (D) Lpl mRNA in BAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. * Statistically significant compared to mice of equal genotype at 28°C or between groups as indicated by bars, according to two-way ANOVA followed by a post-hoc Tukey HSD test (p<0.05). Error bars represent ± SEM. n = 8–10 mice per group.
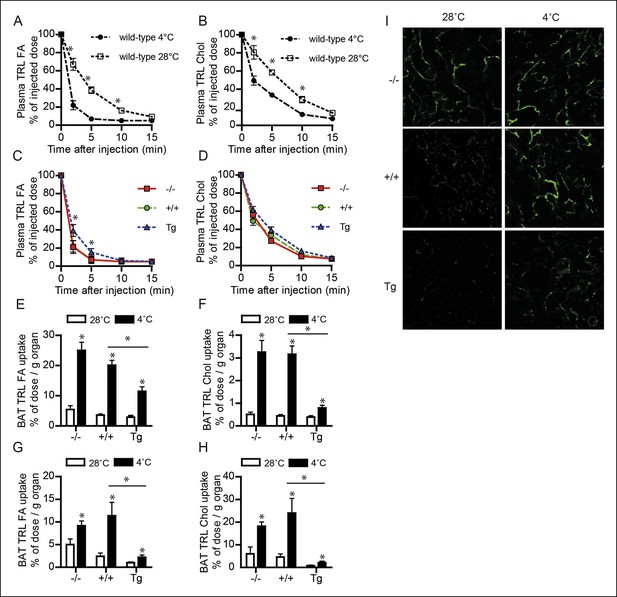
Down-regulation of ANGPTL4 in BAT upon sustained cold exposure promotes an increase in TRL-derived fatty acid uptake by BAT.
(A,B) Plasma 3H (A) and 14C (B) activity in wild-type mice exposed to 4°C or 28°C for 10 days intravenously injected with VLDL-like particles labelled with glycerol tri[3H]oleate (TRL FA) and [14C]cholesteryl-oleate (TRL Chol). (C,D) Plasma 3H (C) and 14C (D) activity in Angptl4-/-, wild-type and Angptl4-Tg mice intravenously injected with VLDL-like emulsion particles labelled with glycerol tri[3H]oleate (TRL FA) and [14C]cholesteryl-oleate (TRL Chol), following exposure to 4°C for 10 days. (E,F) 3H activity (E) and 14C activity (F) in interscapular BAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days and intravenously injected with VLDL-like particles labelled with glycerol tri[3H]oleate (TRL FA) and [14C]cholesteryl-oleate (TRL Chol). (G,H) 14C and 3H activity in interscapular BAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days and intravenously injected with chylomicron-like particles labelled with glycerol tri[14C]oleate (TRL FA) (G) and [3H]cholesteryl-oleyloleate (TRL Chol). (I) Fluorescent image of uptake of intravenously injected QD-TRLs into BAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 9 days. Image was taken 12 min post-injection. n = 2 mice per group.* Statistically significant compared to mice of equal genotype at 28°C or between groups as indicated by bars, according to two-way ANOVA followed by a post-hoc Tukey HSD test (p<0.05). Error bars represent ± SEM. n = 7 mice per group, unless otherwise indicated.
(A,B) Schematic representation of clearance studies in Angptl4-/-, wild-type and Angptl4-Tg mice, consisting of 10 days of cold exposure or thermo-neutrality, followed by measurement of triglyceride clearance via injection of either radiolabelled VLDL-like particles or chylomicron-like particles.
Mice were sacrificed 20 min post-injection to analyze the distribution of the radioactive labels.
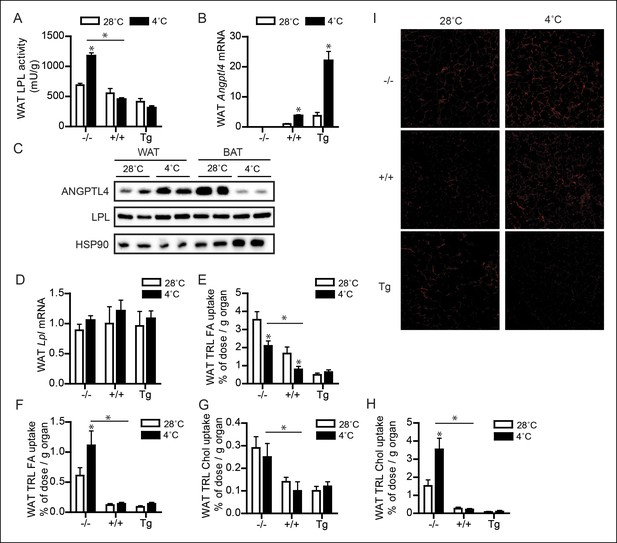
Up-regulation of ANGPTL4 in WAT upon sustained cold exposure suppresses WAT LPL activity and TRL-derived fatty acid uptake.
(A) Total LPL activity levels and (B) Angptl4 mRNA in WAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (C) Immunoblot for ANGPTL4 and LPL in WAT homogenates of wild-type mice exposed to 4°C or 28°C for 10 days. (D) Lpl mRNA in WAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days. (E–H) Activity of 3H and 14C radiolabels in WAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days and intravenously injected with radiolabelled VLDL-like (E,G) and chylomicron-like particles (F,H). TRL FA uptake (E,F) reflects uptake of glycerol tri[3H/14C]oleate, whereas TRL Chol uptake (G,H) reflects uptake of the core labels [14C]cholesteryl-oleate or [3H]cholesteryl-oleyloleate. (I) Fluorescent image of uptake of intravenously injected QD-TRLs into WAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 9 days. Image was taken after perfusion of mice with PBS containing 50 IU/mL heparin and upon cryosectioning of tissues. n = 2 mice per group. * Statistically significant compared to mice of equal genotype at 28°C or between groups as indicated by bars, according to two-way ANOVA followed by a post-hoc Tukey HSD test (p<0.05). Error bars represent ± SEM. n = 7–10 mice per group, unless otherwise indicated.
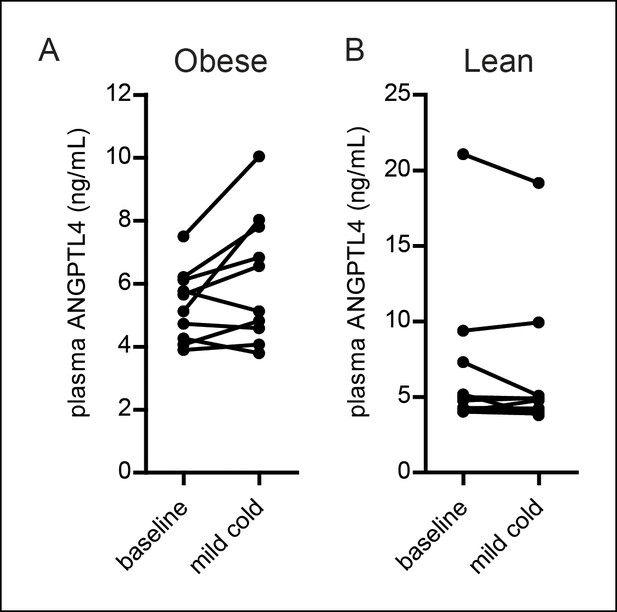
(A,B) Plasma ANGPTL4 levels in 10 obese (A) and 10 lean (B) individuals before and after exposure to a mild cold (16°C) for 48 hr (Wijers et al., 2010).
Differences between mild cold and baseline were statistically significant in the obese group (paired Student’s t-test (p<0.05)). n = 10 individuals per group.
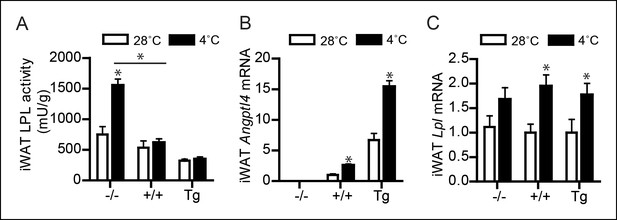
(A) Total LPL activity levels, (B) Angptl4 mRNA, and (C) Lpl mRNA in inguinal WAT (iWAT) of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days.
*Statistically significant compared to mice of equal genotype at 28°C or between groups as indicated by bars, according to two-way ANOVA followed by a post-hoc Tukey HSD test (p<0.05). Error bars represent ± SEM. n = 7–10 mice per group.
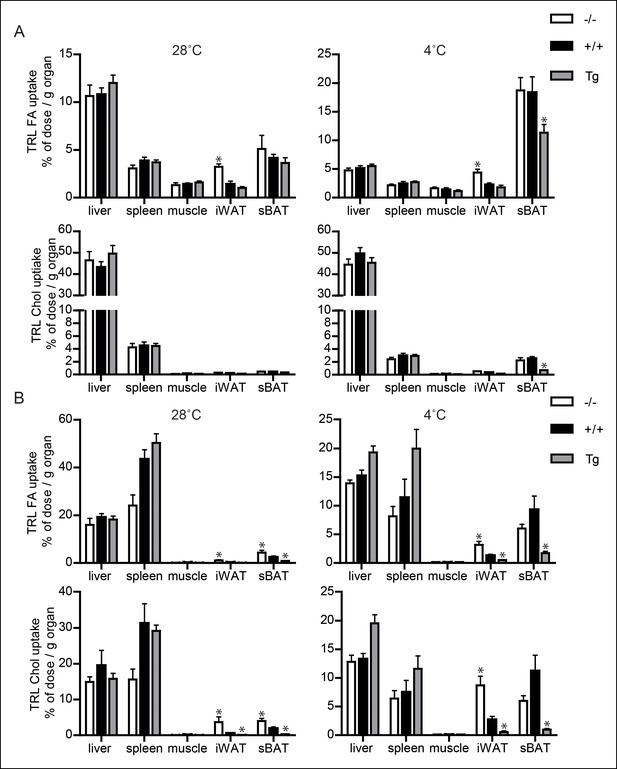
Uptake of TRL-like particles in liver, spleen and muscle is not affected by Angptl4 genotype.
(A) 3H and 14C activity in liver, spleen, muscle, inguinal WAT (iWAT) and subscapular BAT (sBAT) of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days and intravenously injected with VLDL-like emulsion particles labelled with glycerol tri[3H]oleate (TRL FA) and [14C]cholesteryloleate (TRL Chol). (B) 3H and 14C activity in liver, spleen, muscle, iWAT and sBAT of Angptl4-/-, wild-type and Angptl4-Tg mice exposed to 4°C or 28°C for 10 days and intravenously injected with chylomicron-like particles labelled with glycerol tri[14C]oleate (TRL FA) and [3H]cholesteryl-oleyloleate (TRL Chol). *Statistically significant compared to values of wild-type mice according to Student’s t-test (p<0.05). Error bars represent ± SEM. n = 7 mice per group.
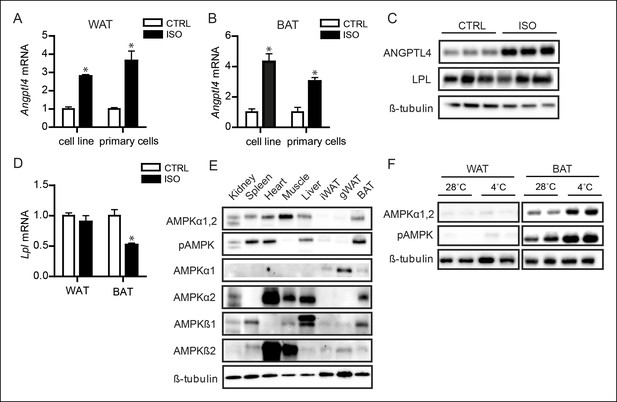
AMPK is activated in BAT, but not WAT, upon sustained cold exposure.
(A) Angptl4 mRNA in differentiated mouse white adipocytes (primary adipocytes and 3T3F442a adipocytes) upon treatment with 10 μM isoproterenol (ISO) or control (CTRL) for 3 hr. (B) Angptl4 mRNA in differentiated brown adipocytes (primary adipocytes and T37i adipocytes) treated with 10 μM isoproterenol (ISO) orcontrol (CTRL) for 3 hr. (C) Immunoblot for ANGPTL4 and LPL protein in differentiated 3T3F422a cells treated with 10 μM isoproterenol (ISO) or control (CTRL) for 3 hr. (D) Lpl mRNA in differentiated mouse primary white or brown adipocytes upon treatment with 10 μM isoproterenol (ISO) or control (CTRL) for 3 hr. (E) Immunoblot for AMPKα1,2 and phospho-AMPK Thr172, AMPKα1, AMPKα2, AMPKβ1 and AMPKβ2 in tissue lysates of kidney, spleen, heart, muscle, liver, inguinal WAT, gonodal WAT and BAT. Homogenates are identical to the homogenates presented in Figure 1A. (F) Immunoblot for AMPKα1,2 and phospho-AMPK Thr172 in BAT and WAT lysates of wild-type mice exposed to 4°C or 28°C for 10 days. *Statistically significant compared to control samples or between indicated treatments according to Student’s t-test (p<0.05). Error bars represent ± SEM.
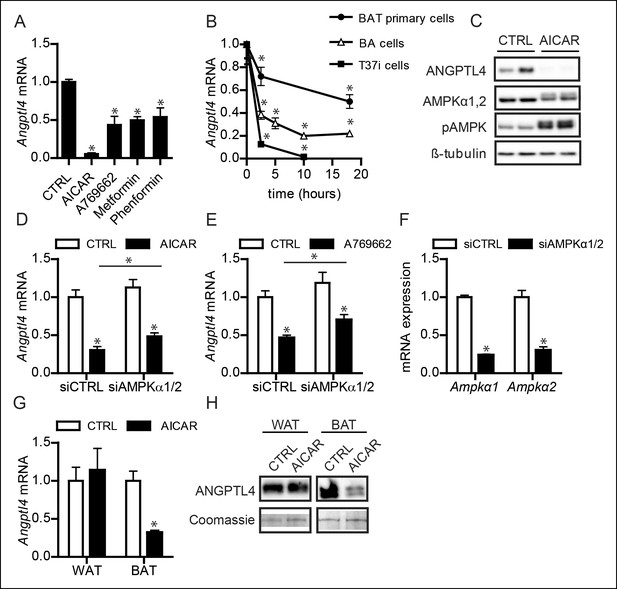
Activation of AMPK down-regulates ANGPTL4 expression specifically in brown adipocytes.
(A) Angptl4 mRNA in differentiated T37i adipocytes treated for 6 hr with 1 mM AICAR, 100 μM A769662, 1 mM metformin or 250 μM phenformin hydrochloride. (B) Angptl4 mRNA in differentiated primary brown adipocytes, BA adipocytes, or T37i adipocytes treated for indicated times with 1 mM AICAR. (C) Immunoblot for ANGPTL4, LPL, AMPKα1,2 and phospho-AMPK Thr172 in differentiated T37i cells treated with control (CTRL) or 1 mM AICAR for 3 hr. (D) Angptl4 mRNA in differentiated T37i adipocytes treated with CTRL siRNA or siRNA against AMPKα1 and AMPKα2 for 48 hr, followed by incubation with control (CTRL) or 1 mM AICAR for 3 hr. (E) Angptl4 mRNA in differentiated T37i adipocytes treated with CTRL siRNA or siRNA against AMPKα1 and AMPKα2 for 48 hr, followed by incubation with H2O control medium (CTRL) or 100 μM A769662 for 6 hr. (E) Ampkα1 and Ampkα2 mRNA in differentiated T37i adipocytes treated with CTRL siRNA or siRNA against AMPKα1 and AMPKα2 for 48 hr. (F) Angptl4 mRNA levels in BAT and WAT explants from C57BL/6J wild-type mice (∼50 μg) treated with H2O control medium (CTRL) or 1 mM AICAR for 3 hr. (G) Immunoblot for ANGPTL4 in BAT and WAT explants from C57BL/6J wild-type mice (∼50 mg) treated with H2O control medium (CTRL) or 1 mM AICAR for 3 hr. *Statistically significant compared to control samples or between indicated treatments, according to Student’s t-test (p<0.05). Error bars represent ± SEM.
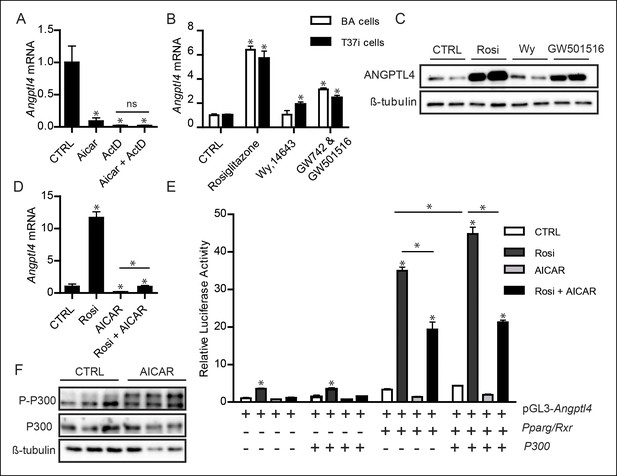
Down-regulation of Angptl4 expression by AMPK is likely mediated via inhibition of PPARγ-mediated transcription of Angptl4.
(A) Angptl4 mRNA in differentiated T37i adipocytes pre-incubated with 0.5 μg/mL actinomycin D (ActD) or DMSO control for 1 hr and treated with 1 mM AICAR or control for 3 hr. (B) Angptl4 mRNA in differentiated T37i or BA adipocytes treated with DMSO control, 5 μM rosiglitazone, 10 μM Wy14643, or 5 μM GW742 (BA adipocytes) or 5 μM GW501516 (T37i adipocytes) for 6 hr. (C) Immunoblot for ANGPTL4 in differentiated T37i adipocytes treated with 5 μM rosiglitazone (Rosi), 10 μM Wy14643 (Wy), and 5 μM GW501516 for 24 hr. (D) Angptl4 mRNA in differentiated T37i cells treated with DMSO control, 5 μM rosiglitazone (Rosi), 1 mM AICAR or both rosiglitazone and AICAR for 6 hr. (E) Relative luciferase activity of HepG2 cells transfected with pGL3-Angptl4, pSG5-Pparγ, pSG5-Rxr, pcDNA-P300HA vectors, as indicated and treated 16 hr post-transfection with 5 μM rosiglitazone, 1 mM AICAR or both compounds for 9 hr. Data are represented as ± SD. (F) Immunoblot of P300 and phospho-P300 (Ser-89) in differentiated T37i brown adipocytes treated with control or 1 mM AICAR for 3 hr. *Statistically significant compared to control samples or between indicated samples according to Student’s t-test (p<0.05). Error bars represent ± SEM, unless otherwise specified.
Tables
Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| m36b4 | ATGGGTACAAGCGCGTCCTG | GCCTTGACCTTTTCAGTAAG |
| mAngptl4 | GTTTGCAGACTCAGCTCAAGG | CCAAGAGGTCTATCTGGCTCTG |
| mLpl | GGGAGTTTGGCTCCAGAGTTT | GGGAGTTTGGCTCCAGAGTTT |
| mUcp1 | CCTGCCTCTCTCGGAAACAA | TGTAGGCTGCCCAATGAACA |
| mPgc1α | AGTCCCATACACAACCGCAGTCGCAACATG | CCCTTTCTTGGTGGAGTGGCTGCCTTGG |
| mCidea | TGACATTCATGGGATTGCAGAC | GGCCAGTTGTGATGACTAAGAC |
| mElovl3 | TTCTCACGCGGGTTAAAAATGG | GAGCAACAGATAGACGACCAC |
| mPrdm16 | CCACCAGCGAGGACTTCAC | GGAGGACTCTCGTAGCTCGAA |





