Transmembrane chemokines act as receptors in a novel mechanism termed inverse signaling
Figures
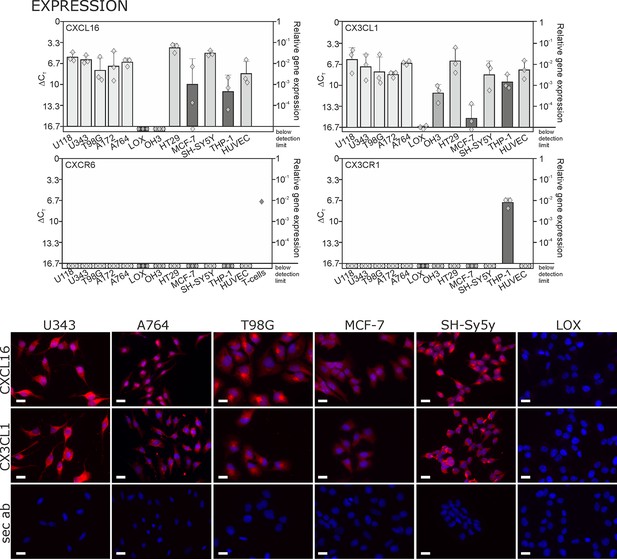
Expression of transmembrane chemokines and their known receptors in various cell types.
Top: As determined by qRT-PCR, the transmembrane chemokines CXCL16 and CX3CL1 are highly transcribed in many human tumor cell lines including glioma (U118, U343, T98G, A172, A764), colon carcinoma (HT29)and neuroblastoma cells (SH-SY5Y), in monocytes (THP-1) and in endothelial cells (HUVEC), at lower levels in breast cancer cells (MCF-7), but not/negligible in LOX melanoma. OH3 small cell lung cancer cells produced CX3CL1, but not CXCL16. In contrast, the known receptors CXCR6 or CX3CR1 were only detectable in a sample of activated T cells or in THP-1 cells, but not in tumor or endothelial cells (n = 3 biological replicates, single data indicated by diamonds). Bottom: Immunostaining of a selection of tumor cells exemplarily confirms cell specific protein expression levels of the transmembrane chemokines, and their absence in LOX melanoma cells. Micrographs were taken with exposure times of 600 ms (CXCL16) or 800 ms (CX3CL1, secondary antibody control [sec ab]) for each cell line. Bars indicate 20 µm, n = 3 independent experiments.
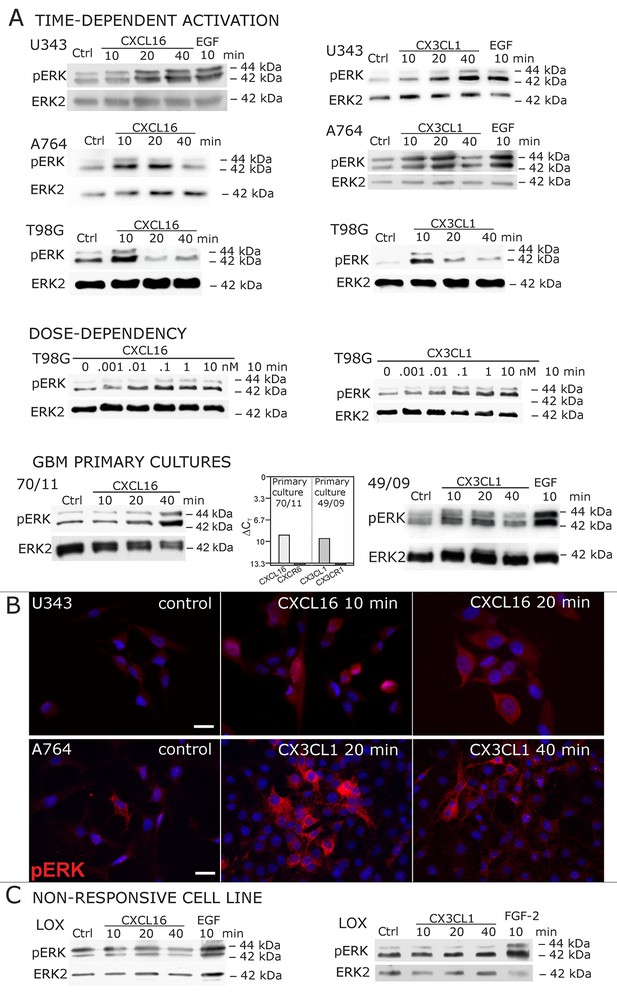
Signal transduction in receptor-negative (CXCR6-, CX3CR1-) tumor cells after stimulation with soluble chemokines (1 nM s-CXCL16 or s-CX3CL1).
A) As shown by Western blots after SDS-PAGE separation, receptor-negative but hence responsive cell lines like the glioma cell lines U343, A764, T98G and primary glioma cultures from different patients display a time- and dose-dependent phosphorylation of the kinase ERK (extracellular signal-regulated kinase p42/p44) after stimulation with s-chemokines for the indicated times (compare also Figure 2 - figure supplement 1). The responsiveness coincidences with the presence or absence of the corresponding tm-chemokines; compare Figure 1. Stimulation with epidermal growth factor (EGF; 2 nM) serves as a positive phosphorylation control. Re-blot against non-phosphorylated kinase ERK2 ensures equal loading of the lanes. (B) Immunostaining of s-chemokine-stimulated U343 or A764 glioma cells confirms the time-dependent phosphorylation of ERK (rabbit anti-pERK 1/2 with secondary antibody Alexa-Fluor 555 (red)-anti-rabbit IgG; blue nuclear counterstaining with DAPI). Bars indicate 20 µm. (C): The tm-chemokine negative LOX melanoma cells are not responsive to 1 nM s-chemokines, ERK-phosphorylation is only observed in positive control samples stimulated with epidermal or fibroblast growth factors (EGF or FGF-2, 2 nM). All shown data are representative results from 2-3 independent experiments, respectively, for biological replicates please refer to Figure 2—figure supplements 1 and 2.
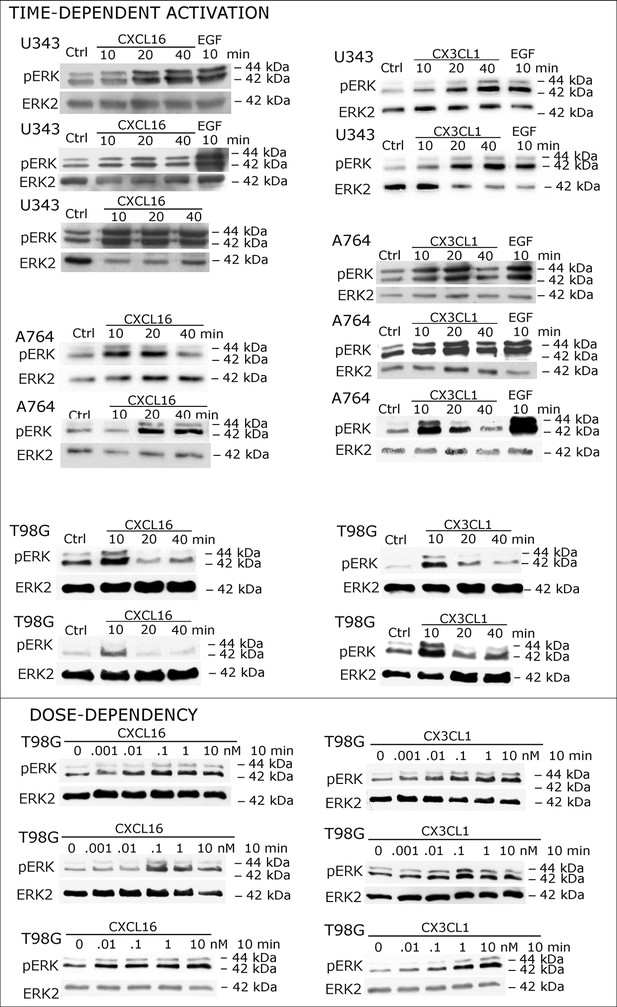
Biological replicates of western blot experiments showing time- and dose-dependent activation of ERK1/2 upon stimulation with s-chemokines in responsive glioma cell lines (compare Figure 2A)
https://doi.org/10.7554/eLife.10820.005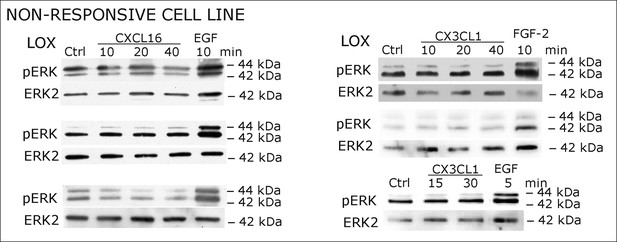
Biological replicates of western blot experiments showing stimulations of non-responsive LOX melanoma cells with s-chemokines (compare Figure 2C)
https://doi.org/10.7554/eLife.10820.006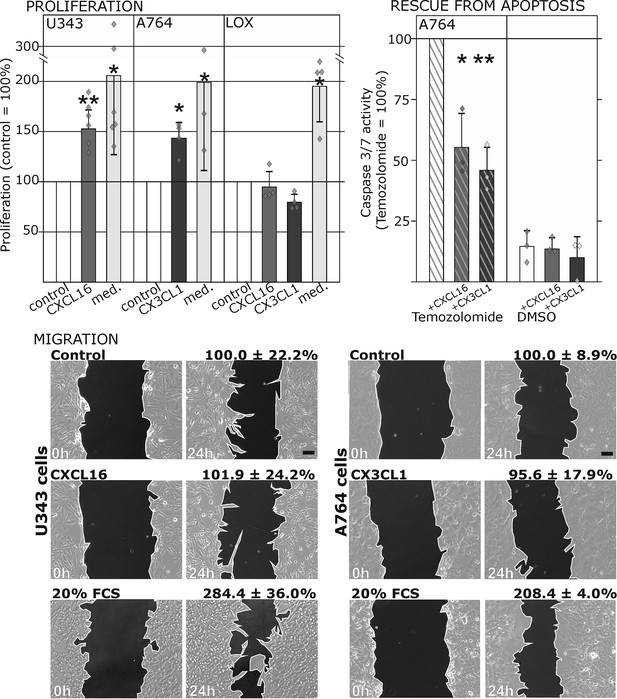
Biological effects after stimulation of receptor-negative (CXCR6-, CX3CR1-) tumor cells with soluble chemokines (1 nM s-CXCL16 or s-CX3CL1).
Top: Soluble chemokines (1 nM) enhance proliferation of U343 and A764 glioma cells expressing the transmembrane counterparts, but not of LOX melanoma cells that are tm-chemokine negative. Stimulation was performed for 24 hr and proliferation analyzed by WST-assay. As positive control, growth medium (med.) with 10% fetal calf serum (FCS) was used. Mean values ± standard deviations of at least 3 independent biological replicates (indicated as diamonds) are shown. Moreover, both s-chemokines (1 nM, respectively) reduced caspase-3/7 activity evoked by the chemotherapeutic Temozolomide (400 µg/ml, from stock solution in dimethylsulfoxide, DMSO). Stimulations were performed for 48 hr, controls were supplemented with 2% DMSO (corresponding to the solvent concentration in Temozolomide-stimulated samples). Caspase 3/7 activity was measured by fluorescence of the converted substrate in 3 independent biological replicates. Mean values ± standard deviations are shown. For effects of the tm-chemokine low expressing cell line compare Figure 3—figure supplement 1. Bottom: Migration of U343 and A764 glioma cells was not influenced by stimulation with 10 nM s-CXCL16 or s-CX3CL1 in a wound healing (‘scratch’) assay performed for the indicated times. 20% FCS served as positive control. Mean values ± standard deviations are shown from 2-3 independent biological replicates, for data of biological replicates compare Figure 3—source data 1. Representative images are shown, bars indicate 50 µm.
-
Figure 3—source data 1
Biological replicates of the scratch assay shown in Figure 3.
- https://doi.org/10.7554/eLife.10820.008
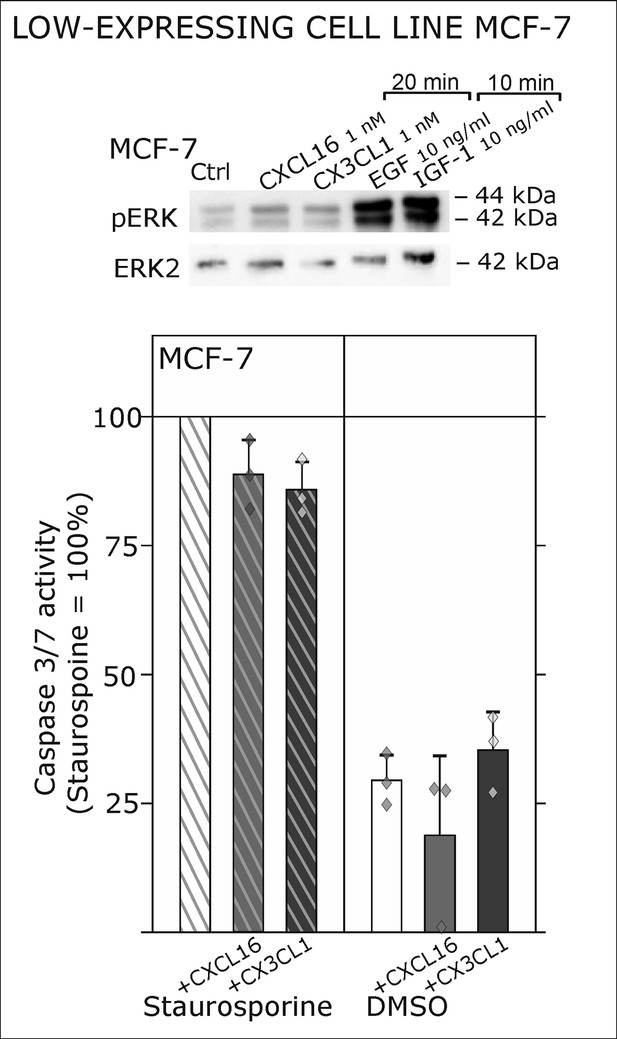
Only slight activation and effects upon s-chemokine stimulation of tm-chemokine low expressing MCF-7 cells.
Top: MCF-7 breast carcinoma cells were stimulated with 1 nM s-CXCL16 or s-CX3CL1 for 20 min and subjected to Western blot on ERK1/2 phosphorylation. In comparison to positive controls (EGF and IGF-1, 10 min) s-chemokines exerted only slight activation of the MAP kinase pathway. In accordance, caspase 3/7 activity induced by 0.1 µM Staurosporine (15 hr) wasis only slightly reduced by co-stimulation with s-chemokines, and hardly robust (n = 3 independent experiments).
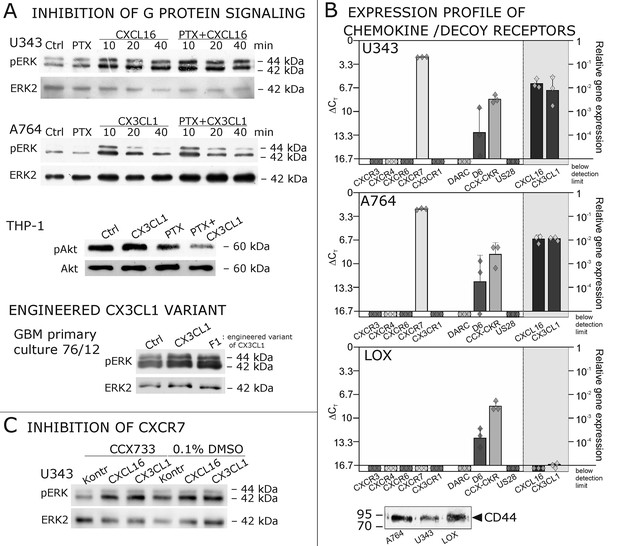
Inhibition experiments and transcription analysis exclude the involvement of other chemokine receptors.
(A) Pertussis toxin (PTX, 200 ng/ml) inhibiting Gi/o-signaling of classical chemokine receptors has no effect on s-chemokine-mediated phosphorylation of kinases in U343 or A764 cells. However, in CX3CR1-expressing THP-1 cells (compare Figure 1) CX3CL1 mediated phosphorylation of Akt (stimulation with 1 nM for 20 min) can be inhibited by pre-incubation with PTX. An engineered variant of CX3CL1, the recombinant CX3CR1-antagonist F1 (100 nM, 20 min) induces also signal transduction in primary glioma cells indicating a mechanism different from CX3CR1-binding (and antagonism). Shown are representative Western blots after SDS-PAGE separation of lysates from stimulated U343 or A764 glioma cells stained for pERK 1/2 or pAkt (re-blot to non-phosphorylated kinases, control of equal loading) from 3 independent experiments, compare Figure 4—figure supplement 1. (B) The transcription profile of classical chemokine receptors and chemokine decoy receptors as determined by quantitative RT-PCR shows that the chemokine receptors CXCR3, CXCR4, CXCR6 and CX3CR1 are absent in responsive U343 and A764 and non-responsive LOX cells. However, the atypical chemokine receptor CXCR7 that is known to signal G protein-independently is expressed in responsive cell lines and absent in LOX cells. The chemokine decoy receptors D6 and CCX-CKR are expressed at comparable levels in responsive and non-responsive cells, whereas DARC is absent (n = 3 biological replicates, indicated by diamonds). Additionally, the cytomegalovirus-derived gene US28 that encodes for a putative CX3CR1 receptor could not be detected in these cell lines. The highly glycosylated protein CD44, that may putatively sequester chemokines, was detected at comparable protein levels in U343, A764 and LOX cells (n = 3). (C) To investigate the contribution of CXCR7 in s-chemokine mediated signaling U343 cells were pre-incubated with the CXCR7-antagonist CCX733 (100 nM, 2 hr) and stimulated with 1 nM of s-CXCL16 or s-CX3CL1 for 20 min. Controls ± s-chemokines were pre-incubated with 0.1% DMSO as solvent controls. The CXCR7-antagonist CCX733 does not impair s-chemokine-mediated ERK phosphorylation. Representative Western blots from 3 independent experiments. For biological replicates of western blots please refer to Figure 4—figure supplement 1.
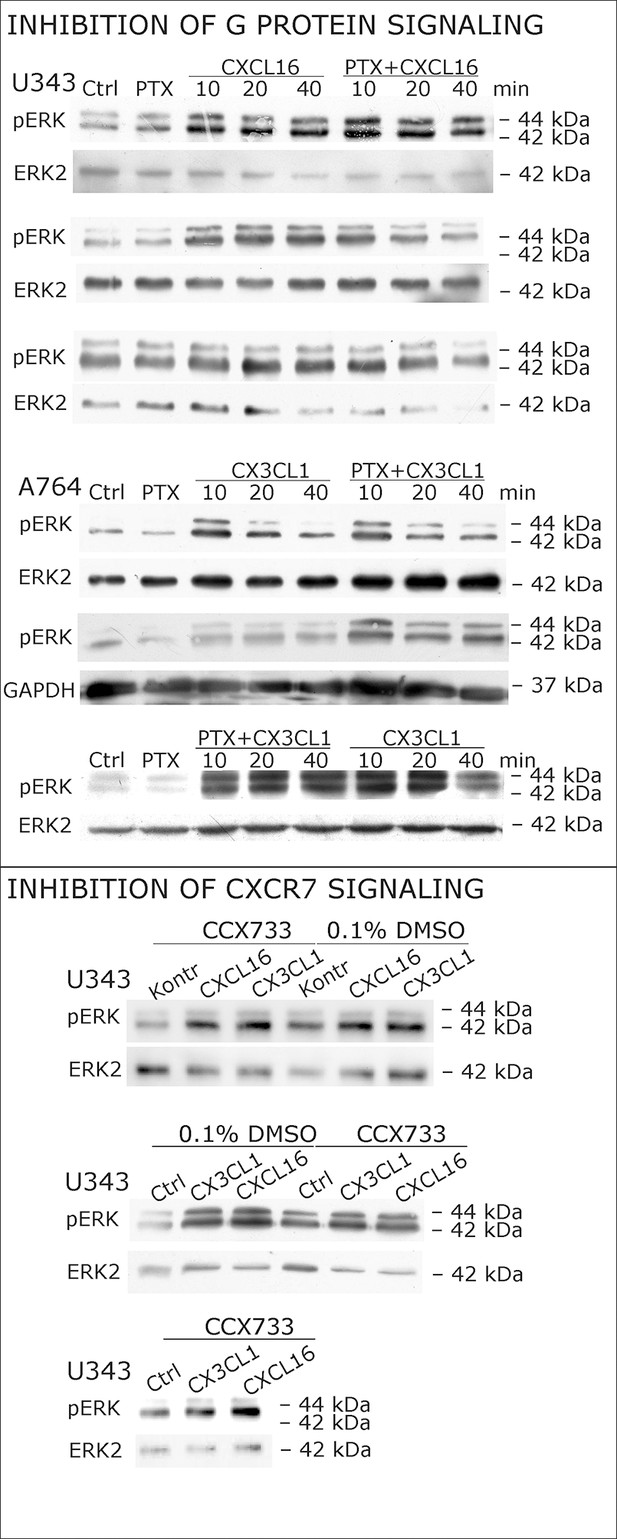
Biological replicates of western blot experiments with s-chemokines and Pertussis toxin (compare Figure 4A).
https://doi.org/10.7554/eLife.10820.011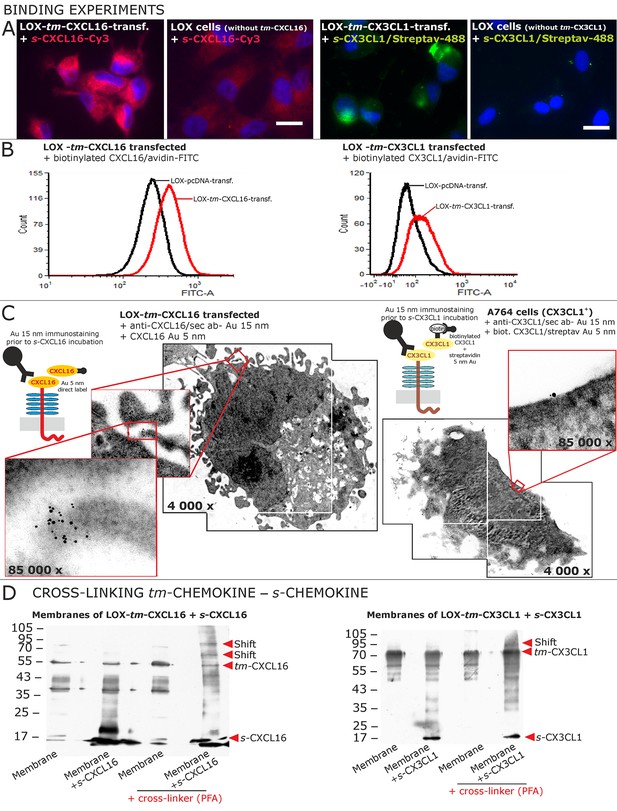
Binding of soluble chemokines to corresponding tm-chemokines on cell surfaces.
(A) Fluorescent soluble chemokines, either labeled directly (cyanine3, Cy3) or indirectly via biotin-label to fluorescent (strept)avidin (Alexa-Fluor 488 or fluorescein-isothiocyanate, FITC), bind to tumor cells expressing tm-chemokines (transfected LOX melanoma, or glioma cells, not shown), but not to non-transfected LOX cells as shown by light microscopy. Due to its lipophilic character, the dye Cy3 yields a whiff of background. However, this was also observed with the negative control Cy3-labeled lactalbumin. Chemokine concentrations 2 nM, bars indicate 20 µm. (B) FACS analysis confirmed binding signals yielded by fluorescence microscopy. LOX cells transfected with tm-CXCL16 were detectable by their binding of biotinylated CXCL16 (2 nM)/Avidin-FITC, whereas mock transfected cells (LOX-pcDNA) were not, and LOX cells transfected with tm-CX3CL1 were labeled by biotin-CX3CL1 (2 nM)/Avidin-FITC, in comparison to mock transfected cells. (C) A close association between s- and tm-chemokines is visible by immuno-electron microscopy of tm-chemokine-expressing/-transfected cells which were immunolabeled with anti-CXCL16 or CX3CL1 and 15 nm-gold-labeled secondary antibodies and subsequently incubated with 5 nm-gold-labeled (directly or via biotin/streptavidin complex) soluble chemokines (2 nM) before embedding. (D) Chemical cross-linking by paraformaldehyde (PFA) shifts tm-chemokine bands to higher molecular weights. Isolated membranes of tm-chemokine overexpressing LOX cells were incubated over night with 2 nM s-chemokines, cross-linked with 1% PFA and subjected to SDS-PAGE separation and subsequent Western blotting using antibodies against the respective chemokine domains of CXCL16 and CX3CL1. All experiments were repeated in 2 independent biological replicates, and representative photographs and immunoblots are shown.
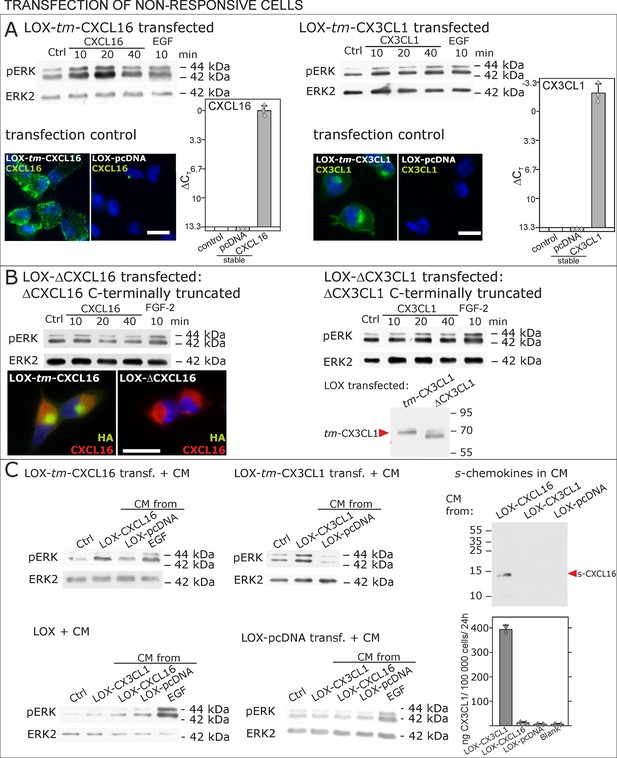
Non-responsive cells can be transformed to be responsive to s-chemokine stimulation by transfection with tm-chemokines.
(A) LOX melanoma cells were stably transfected with tm-chemokines. Transfection efficiency was controlled by quantitative RT-PCR and immunocytochemistry (n = 3 biological replicates as indicated by diamonds; bars represent 20 µm). Cells were then stimulated with s-chemokines (1 nM) and cell lysates analyzed by SDS-PAGE separation and immunoblotting for phosphorylation of ERK 1/2 (re-blots for ERK2 ensure equal loading). Transfected cells responded with ERK 1/2-phosphorylation in contrast to non-transfected cells (compare Figure 2). (B) Stably transfected LOX-cells expressing C-terminally truncated tm-chemokine variants lacking the intracellular domain (LOX-ΔCXCL16 and LOX-ΔCX3CL1) cannot be activated by stimulation with s-chemokines (1 nM). Thus, the intracellular domain of the tm-chemokines seems to be critical for signaling. Successful truncation was proven by co-immunostaining with anti-CXCL16 (extracellular chemokine domain, red) and anti-HA (intracellular tag of the CXCL16-expression vector, green), or band shift in Western blot. To obtain defined bands, proteins were deglycosylated prior to SDS-PAGE. (C) Shed chemokine domains found in conditioned media (CM) from overexpressing cells can also mediate activation of tm-chemokine transfected LOX cells. Conditioned media were obtained from confluent CXCL16 or CX3CL1 or mock (pcDNA) transfected cells (LOX-CXCL16, LOX-CX3CL1, LOX-pcDNA) and applied to tm-chemokine expressing LOX cells for 20 min. SDS-PAGE plus immunoblotting (CXCL16) or ELISA (CX3CL1) proved presence of shed chemokines in the conditioned media used for stimulation. Stimulation with conditioned media containing shed chemokine domains can activate the ERK signaling in tm-chemokine transfected cells, but not in pcDNA-transfected or not modified LOX cells. All shown results are representatives from 3 independent experiments, except for the control of successful truncation of tm-chemokines in stable clones and experiments with conditioned media that were performed twice; for examples of biological replicates compare Figure 6—figure supplement 1.
Biological replicates of western blot experiments with transfected LOX melanoma cells (compare Figure 6).
https://doi.org/10.7554/eLife.10820.014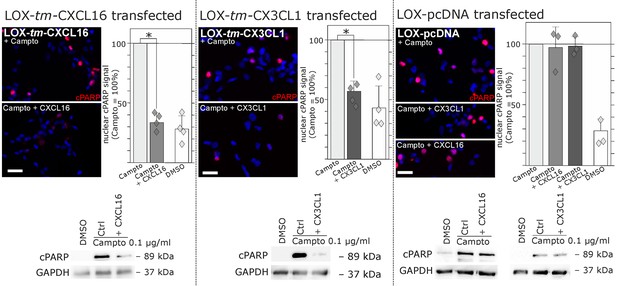
Stimulation with s-chemokines can mediate rescue from chemically-induced cell death in tm-chemokine-transfected LOX melanoma cells.
LOX melanoma cells stably expressing tm-CXCL16 or tm-CX3CL1 (or mock transfected LOX cells) were treated for 18 hr with 0.1 µg/ml Camptothecin (inhibitor of topoisomerase I) to induce cell death. Simultaneous stimulation of tm-CXCL16-LOX with 1 nM s-CXCL16 or tm-CX3CL1-LOX with 1 nM s-CX3CL1 significantly reduced cell death as indicated by reduced cleavage of poly(ADP ribose) polymerase (PARP) (shown by Western blot after SDS-PAGE, n = 2 biological replicates; or immunocytochemistry, n = 3-4 biological replicates, indicated by diamonds). In contrast, mock-transfected (pcDNA) LOX cells did not show reduced signals of cleaved PARP when stimulated with s-CXCL16 or s-CX3CL1. Bars represent 50 µm.
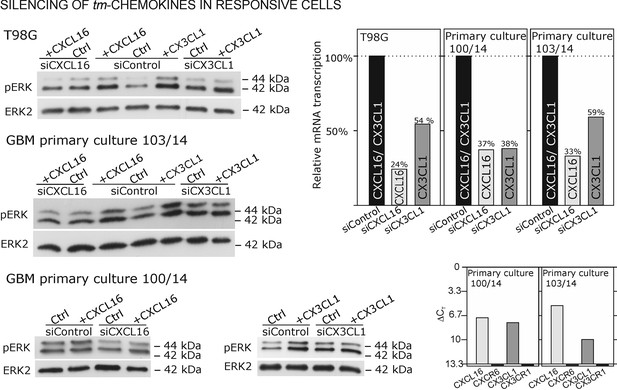
Silencing of tm-chemokine expression abolishes the s-chemokine mediated activation in responsive glioma cell lines and primary cultures.
Cell lines and primary cells were transfected with CXCL16 or CX3CL1 specific RNAi (or non-specific control RNAi), left for recovery for 36 hr and stimulated with 1nM CXCL16 or CX3CL1 for 15 min. Samples were analyzed by SDS-PAGE separation and immunoblotting for phosphorylated ERK 1/2, and re-probed for ERK 2 to ensure equal loading. Silencing efficiency and basic transcription level were confirmed by quantitative RT-PCR (right panel). In tm-chemokine silenced cultures, no activation can be observed by incubation with the respective s-chemokine. Experiments with T98G were repeated in 2 independent biological replicates, shown primary cultures are representative examples from 2 different patient’s primary cultures (compare Figure 8—figure supplement 1).
Biological replicates of western blot experiments from siRNA knockdown in T98G glioma cells (compare Figure 8).
https://doi.org/10.7554/eLife.10820.017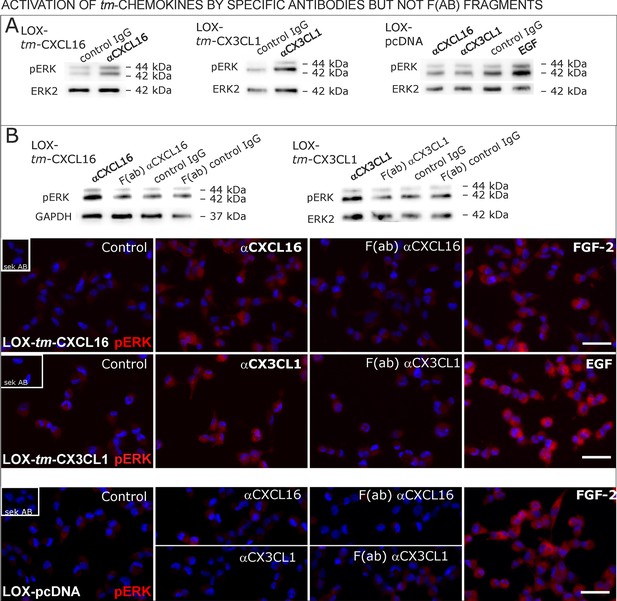
Signal transduction in tm-chemokine expressing LOX cells upon stimulation with specific antibodies (0.1 µg/ml), but not monovalent F(ab) fragments.
(A) As shown by Western blot after SDS-PAGE, stimulation of tm-CXCL16 or tm-CX3CL1 transfected LOX cells for 20 min with antibodies against the corresponding chemokine domains (0.1 µg/ml), yields a phosphorylation signal for ERK1/2. Non-specific control IgG could not elicit signaling, nor could specific antibodies activate mock-transfected (LOX-pcDNA) cells (positive stimulation control: 2 nM FGF-2, n = 2-3 biological replicates, for corresponding effects in glioma cells compare Figure 9—figure supplement 1). (B) In contrast to the intact specific antibodies, monovalent F(ab) fragments (0.1 µg/ml) obtained by papain digestion and clean-up of these fragments failed to mediate ERK1/2 phosphorylation as demonstrated by Western blot and immunocytochemistry (FGF-2 serves as positive control, n = 3 biological replicates, Bars represent 50 µm).
Biological replicates of western blot experiments of stimulations with chemokine-specific antibodies (compare Figure 9).
https://doi.org/10.7554/eLife.10820.019Tables
Sequences of putative intracellular domains from transmembrane chemokines.
| CX3CL1 |
Homo sapiens (Human) | -QSLQGCPRKMAGEMAEGLRYIPRSCGSNSYVLVPV |
| Nomascus leucogenys (Northern white-cheeked gibbon) | -QSLQGCPRKMAGEMAEGLRYIPRSCGSNSYVLVPV |
| Macaca mulatta (Rhesus macaque) | -QSLQGCP RKMAGEMVEGLRYIPRSCGS NSYVLVPV |
| Bos taurus (Bovine) | -QRLQSCPHKMVGDVVEGICYVPRSCGSNSYVLVPV |
| Canis familiaris (Dog) | -YQSLQGCSR KMAGDMVEGLRYVPRSCGSN SYVLVPV |
| Oryctolagus cuniculus (Rabbit) | -Q SLQGCPRKMAGEMVEGLRYV PRSCGANSYVLVPV |
| Cavia porcellus (Guinea pig) | -QSLQGCPRK MAGEMVEGLRYVPRSCGSNSYVLVPV |
| Rattus norwegicus (Rat) | -QS LQGCPRKMAG EMVEGLRYVP RSCGSNSYVLVPV |
| Mus musculus (Mouse) | -QSLQGCPRKM AGEMVEGLRYVPRSCGSNSYVLVPV |
| Monodelphis domestica (Gray short-tailed opossum) | -QSLQSCPRRMAGEVVEGLRYIPRSCGSNSYVLVPV |
| Sarcophilus harrisii (Tasman devil) | -QSLQSCPRRMAGEVVEGLRYIPRSCGSNSYVLVPV |
| Ornithorhynchus anatinus (Duckbill platypus) | -QSLQSCPRRMAGEVVEGLRYIPRSCGSNSYVLVPV |
| CXCL16 | |
Homo sapiens (Human) | -CKRRRGQSPQSSPD PVHYIPVAP DSNT |
| Gorilla gorilla gorilla (Lowland gorilla) | -CKRRRGQSPQSSPGLPVHYIPVAPDSNT |
| Nomascus leucogenys (Northern white-cheeked gibbon) | -CKR RRGQSPQSSPDLQFHYIPVA PDSNT |
| Macaca mulatta (Rhesus macaque) | -CKRRGQSPQSSPDLQLHYIPVASDSNT |
| Sus scrofa (Pig) | -CKKRQEQSRQYPPDPQLHYVPVASNINT |
| Loxodonta africana (African elephant) | -CKRRREQSRLYYPDLQFHYKPVA PDS |
| Bos taurus (Bovine) | -C KRRKNQLLQHPPDLAASLYT CSRRTRAENGTL |
| Equus caballus (Horse) | -CKKREKTLRPSPDLQAHYERVAPD |
| Canis familiaris (Dog) | -CKRREQSLQHPPDLQLHYTPVASDSNV |
| Oryctolagus cuniculus (Rabbit) | -CKRRRGRSPKYSSGKP |
| Rattus norwegicus (Rat) | -CNRRVTRQSSSGLQLCYTPV EPRPQGL |
| Mus musculus (Mouse) | -CNRRATQQNSAGLQLWYTPVEPRP |
| Myotis lucifugus (Little brown bat) | -CKRRSKQSPQYSPDLQLQCIPVASYSNS |
| Ornithorhynchus anatinus (Duckbill platypus) | -CRRRGAPRNEMLYPQRPKGTSITVQANSPT |
-
Data from: Uniprot (http://www.uniprot.org/)
-
Putative intracellular domains are depicted by homology to the published putative human sequences.
-
-SXXS- motifs, -SXXT- motifs, -SXS- motifs; -SXPV/R- SH2-binding site





