Soluble Fas ligand drives autoantibody-induced arthritis by binding to DR5/TRAIL-R2
Figures
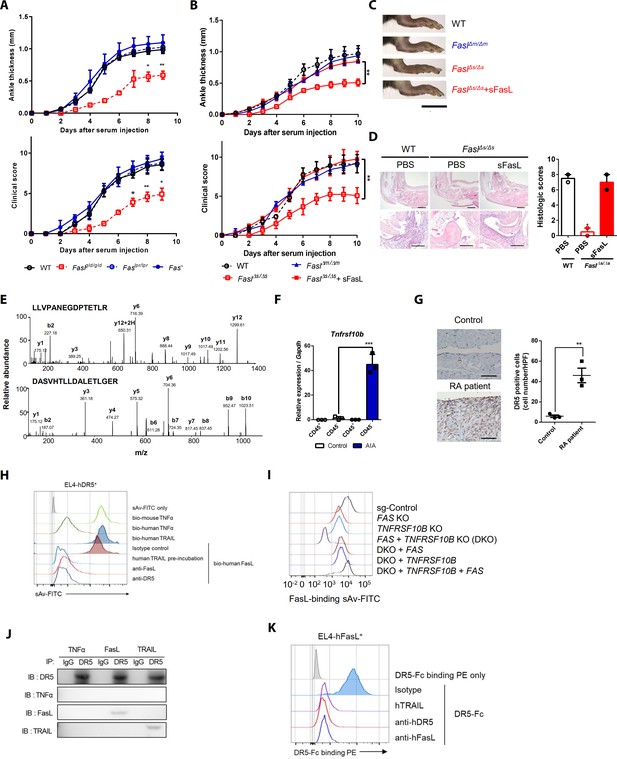
Death receptor (DR5) is a Fas-independent receptor for soluble Fas ligand (sFasL) that promotes arthritis.
(A) Joint swelling and clinical scores in wild-type (WT), Faslpr/lpr, Faslgld/gld, and Fas–/– mice (n = 6 per group). (B) Joint swelling and clinical scores in WT, FaslΔm/Δm, FaslΔs/Δs, and FaslΔs/Δs mice injected with sFasL (n = 6 per group). (C, D) Gross and microscopic examination of arthritis (magnified 10× in the upper panel and 200× in the lower panel). Scale bars: 1 cm (C), 200 μm (D, upper panel), and 100 μm (D, lower panel). (E) Tandem mass spectra of unique DR5 peptides. (F) Transcript levels of Tnfrsf10b in synovial CD45+ immune cells and CD45– non-immune cells from WT mice with or without AIA. (G) Immunohistochemistry of DR5 expression in joint tissue from a healthy control subject and a patient with rheumatoid arthritis (n = 3; magnified 400×, scale bar: 50 μm). (H) Flow cytometric analysis of biotinylated protein binding to EL4 cells transfected with human WT TNFRSF10B preincubated with recombinant hTRAIL, or simultaneously incubated with anti-FasL, or anti-DR5 antibodies. (I) Flow cytometric analysis of biotinylated FasL binding on hFLSCs with FAS and/or TNFRSF10B knockout, and TNFRSF10B and/or FAS overexpression in FAS and TNFRSF10B double knockout (DKO) cells. (J) hLFSCs were preincubated with TNF-α (as a negative control), FasL, or TRAIL and cross–linked with BS3. Lysates from these cells were immunoprecipitated with anti–DR5 or control IgG antibody and immunoblotted with anti-DR5, TNF-α, FasL, or TRAIL antibodies. (K) Flow cytometric analysis of DR5–Fc binding on EL4 cells transfected with human WT FASLG in the presence of recombinant hTRAIL, anti-DR5, or FasL antibodies. Data were pooled from three (A, B, and D–G) or four (H, K) independent experiments and are presented as mean ± standard error of the mean (SEM). *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way analysis of variance (ANOVA).
-
Figure 1—source data 1
Numerical data obtained during experiments represented in Figure 1, Figure 1—figure supplements 1, 3, and 4.
- https://cdn.elifesciences.org/articles/48840/elife-48840-fig1-data1-v1.xlsx

Gross examination and measurement of soluble Fas ligand (sFasL) expression in the joints of mice with autoantibody-induced arthritis (AIA).
(A) Expression of Fas in synovial fibroblasts from wild-type (WT), Faslpr/lpr, Faslgld/gld, and Fas–/– mice with arthritis. (B, C) Gross examination (B) and transcript levels (C) of various cytokines and chemokines in the joints of WT, Faslpr/lpr, Faslgld/gld, and Fas–/– mice with arthritis (n = 6 per group). (D and E) Joint swelling and clinical scores (D) and transcript levels of various cytokines and chemokines (E) in WT mice injected with anti-Fas, anti-FasL, or control antibodies (n = 6 per group). (F) Levels of sFasL in synovial fluids obtained from normal mice and WT, FaslΔm/Δm, and FaslΔs/Δs mice with arthritis (n = 3 per group). (G, H) Joint swelling and clinical scores (G) and transcript levels of various cytokines (H) in joint tissues from Faslgld/gld mice injected with sFasL or PBS (n = 6 per group). (I) Transcript levels of various cytokines in the joints of WT and FaslΔs/Δs mice injected with sFasL or PBS. (J) WT and Fas–/– mice were injected with sFasL or PBS to assess AIA. Joint swelling and clinical scores were measured (n = 3 per group). (K) Joint swelling and clinical scores of Faslgld/gld mice that had splenocytes transplanted from WT or Faslgld/gld mice (n = 6 per group). Data are presented as mean ± standard error of the mean (SEM) of independent experiments (B–E, data were pooled from four independent experiments and analyzed; A, F–H, and K, data were pooled from three independent experiments). *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way analysis of variance (ANOVA).

Schematic diagram showing affinity purification–mass spectrometry (AP–MS) analyses, death receptor (DR) 5 and Fas expression in mouse and human joint cells, and assessment of FasL binding to DR5 using flow cytometry.
(A) Schematic diagram showing AP–MS analyses. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of biotinylated Fc (control) or sFasL–Fc cross-linked protein complexes. The fractions used in in-gel digestion are separated by red lines. (C) Expression of DR5 in CD45+ and CD45– cells from the joint tissues of WT mice with arthritis. (D) Flow cytometry analyses of the expression of Fas and DR5 in human (h) fibroblast-like synovial cells (FLSCs). (E) Flow cytometry analyses of FasL–Fc binding to hFLSCs in the presence of anti-DR5 and/or anti-Fas antibodies. (F, G) Flow cytometry analyses of human DR5 (F) and Fas (G) expression in EL4 cells transfected with human WT tumor necrosis factor receptor superfamily (TNFRSF)10B (F) and FAS (G). (H) Expression of TNFRSF10B and FAS in EL4 mouse T cells transfected with various human genes. (I) Flow cytometry analyses of biotinylated protein binding to EL4 cells transfected with human WT FAS preincubated with recombinant human (h) TNF-related apoptosis-inducing ligand (TRAIL) or treated simultaneously with anti-Fas and DR5 antibodies. (J) Flow cytometry analyses of FasL–Fc binding to hFLSCs after preincubation with recombinant sTRAIL or sFasL. All experiments were performed four times independently.
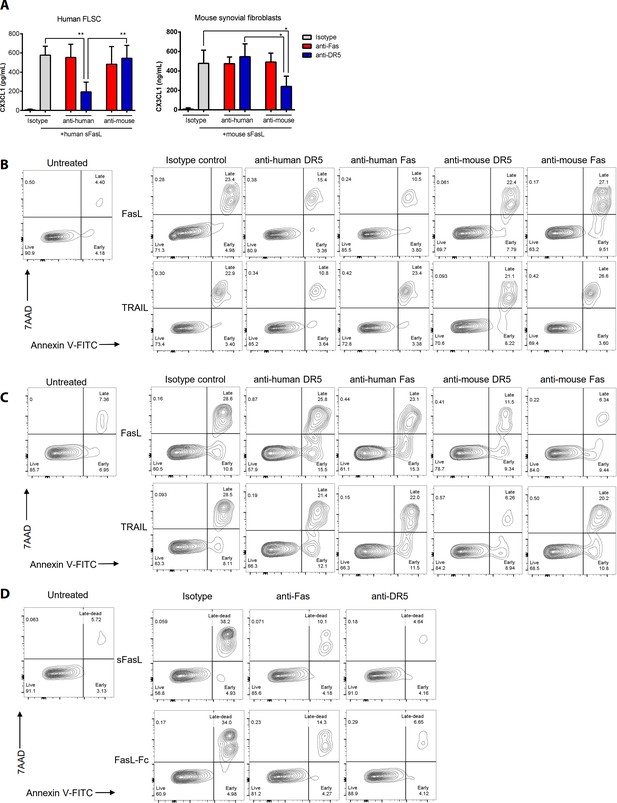
Evaluation of cross-reactivity and functional activity of purchased reagents.
(A) hFLSCs and mouse synovial fibroblasts were stimulated with human or mouse sFasL in the presence or absence of anti-mouse Fas or anti-DR5 antibodies, as well as anti-human Fas or anti-DR5 antibodies for 24 hr. CX3CL1 levels in culture supernatants were measured using ELISA. (B, C) Jurkat cells (B) or mouse splenocytes (C) were incubated for 24 hr with recombinant FasL and TRAIL in the presence or absence of human or mouse anti-DR5 or anti-Fas antibodies. Jurkat and gated splenic TCRβ+ CD4+ T cell death was measured using flow cytometry. (D) Jurkat cell death was measured using flow cytometry after FasL or FasL–Fc treatment in the presence or absence of anti-Fas or anti-DR5 antibodies for 24 hr. Data are presented as mean ± SEM. All experiments were performed three times independently. *p<0.05; **p<0.01. Data were analyzed using one-way ANOVA.
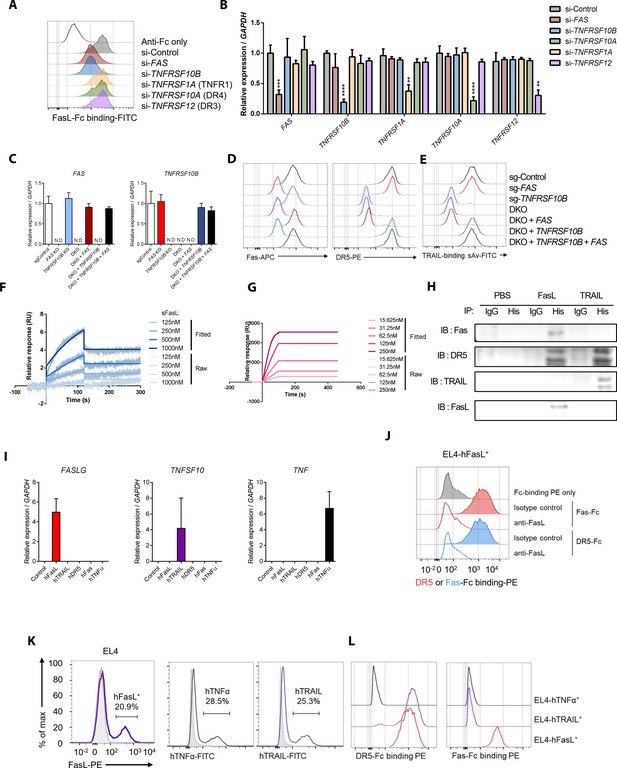
Comparison of sFasL and DR5 binding with that of TRAIL and other TNFRSFs based on flow cytometry, co-immunoprecipitation, and surface plasmon resonance assays.
(A) Flow cytometry analyses of FasL–Fc binding to hFLSCs that were knocked down with FAS, TNFRSF10B, TNFRSF1A, TNFRSF10A, or TNFRSF12. (B) Expression of FAS, TNFRSF10B, TNFRSF10A, TNFRSF1A, and TNFRSF12 in siRNA-transfected hFLSCs. (C) Expression of FAS and TNFRSF10B in FAS- and/or TNFRSF10B-knockout (KO) cells and KO cells transfected with TNFRSF10B and/or FAS overexpression vector. (D, E) Expression of Fas and DR5 (D) and biotinylated TRAIL binding (E) in FAS- and/or TNFRSF10B-KO hFLSCs and DKO (DR5 and Fas gene double knockout) cells transfected with TNFRSF10B and/or FAS in expression vectors. Biotinylated TRAIL binding was quantified by streptavidin (sAv)–fluorescein isothiocyanate staining intensity using flow cytometry. (F, G) Surface plasmon resonance assays for DR5–FasL (F) and DR5–TRAIL (G) interactions. (H) hFLSCs were incubated with PBS (control), 6× His-tagged FasL, or 6× His-tagged TRAIL and cross-linked using BS3. Cell lysates were immunoprecipitated with anti-His or control IgG antibodies and then immunoblotted with anti-Fas, DR5, TRAIL, and FasL antibodies. (I) FASLG, TNFSF10, and TNF expression in EL4 mouse T cells transfected with human genes. (J) Flow cytometry analyses of DR5–Fc and Fas–Fc binding to EL4 cells transfected with human WT FASLG in the presence of anti-FasL antibodies. (K, L) Flow cytometry analyses of human FasL, TNF-α, and TRAIL expression (K), as well as DR5–Fc and Fas–Fc binding to EL4 cells transfected with human WT FASLG, TNFA, or TRAIL (L) and gated on transfected cells expressing the target proteins. Data were pooled from three independent experiments and are presented as mean ± SEM (n = 4 in B). **p<0.01; ****p<0.0005. Data were analyzed using one-way ANOVA.
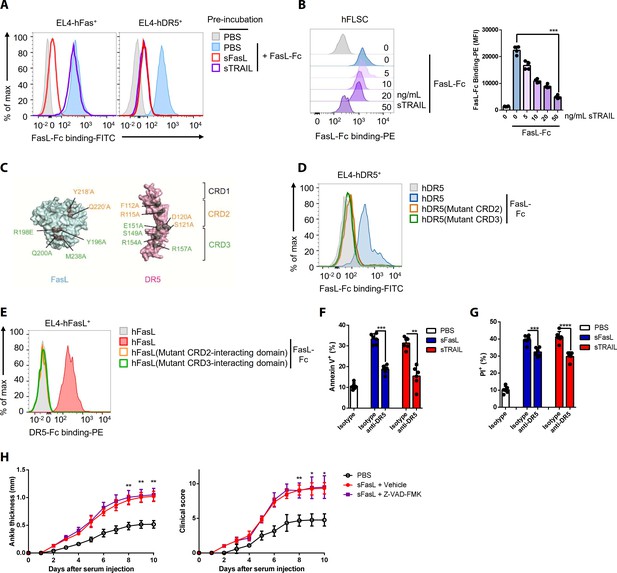
FasL and TRAIL compete for DR5 binding and exert similar effects on cell death.
(A, B) FasL–Fc binding to hFLSCs or EL4 cells transfected with human FAS, or TNFRSF10B after preincubation with human sTRAIL or sFasL. (C) Model of FasL and DR5 derived from the crystal structure of the FasL/DcR3 complex (Protein Data Bank: 4 MSV) and TRAIL/DR5 complex (Protein Data Bank: 1D4V). (D, E) Flow cytometric analysis of FasL–Fc or DR5–Fc binding to EL4 cells transfected with human WT or mutated TNFRSF10B or FASLG. (F, G) Comparison of the effects of sFasL and sTRAIL on (F) apoptosis and (G) necroptosis in hFLSCs. (H) Joint swelling and clinical scores in Faslgld/gld mice injected with Z–VAD–FMK and/or sFasL (n = 6 per group). Data were pooled from four (A, B, and D–G) or three (H) independent experiments and are presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
-
Figure 2—source data 1
Numerical data obtained during experiments represented in Figure 2, Figure 2—figure supplement 3.
- https://cdn.elifesciences.org/articles/48840/elife-48840-fig2-data1-v1.xlsx
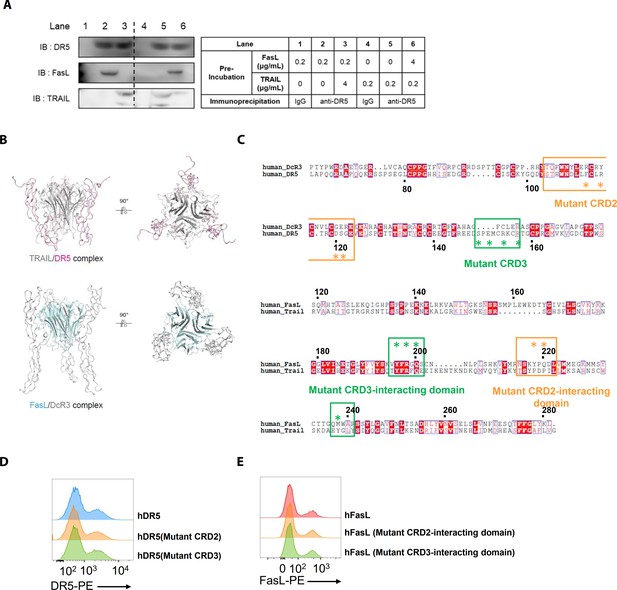
Structural models of the TRAIL/DR5 and FasL/DcR3 complexes, sequence alignments, and transfection efficiencies to investigate interactions involving mutant DR5 and FasL proteins.
(A) hLFSCs were preincubated with FasL and TRAIL using an excess of TRAIL in lane 3 (TRAIL [4 μg/mL]+FasL) and an excess of FasL in lane 6 (FasL [4 μg/mL]+TRAIL) before cross-linking with BS3. Lysates from these cells were immunoprecipitated with anti-DR5 (lanes 2, 3, 5, and 6) or control IgG (lanes 1 and 4) antibodies and immunoblotted with anti-DR5, FasL, or TRAIL antibodies. (B) Crystal structures of the TRAIL/DR5 (Protein Data Bank: 1D4V) and FasL/DcR3 (Protein Data Bank: 4 MSV) complexes. (C) Alignment of the human DcR3 and DR5 as well as the human FasL and TRAIL sequences. The point mutations in the mutant huDR5–cysteine-rich domains (CRD)two and CRD3, the mutant FasL–CRD2 interacting domain, and the mutant FasL–CRD3 interacting domain are indicated by asterisks (*). (D) Flow cytometry analyses of human DR5 in EL4 cells transfected with human WT or mutant TNFRSF10B. (E) Flow cytometry analyses of human FasL in EL4 cells transfected with human WT or mutant FASLG. Experiments (A, D, and E) were performed three times independently.
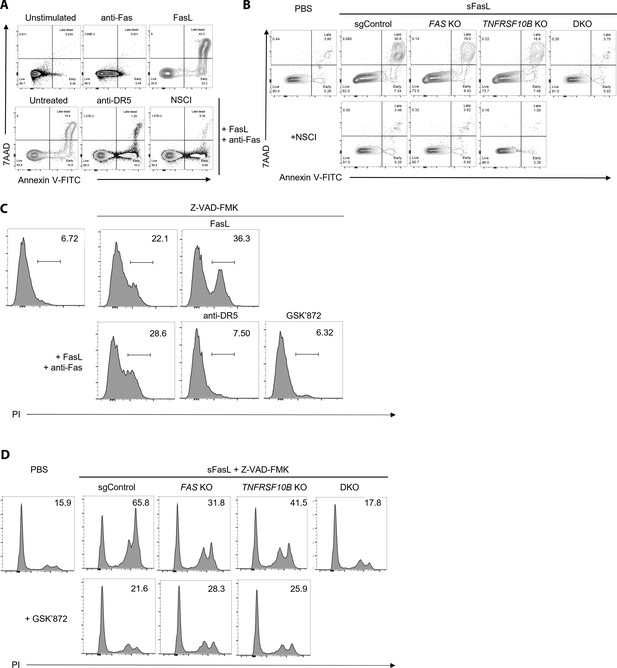
FasL-mediated apoptosis and necroptosis in hFLSCs upon treatment with specific inhibitors WT (A, C) or FAS and TNFRSF10B KO (B, D) hFLSCs were treated with sFasL in the presence of anti-Fas or anti-DR5 antibodies and cell death was quantified.
Apoptosis (A, B) and necroptosis (C, D) were measured using flow cytometry in the presence of specific inhibitors of apoptosis (NSCI) and necroptosis (GSK’872), respectively. The data shown are representative of the results from three independent experiments.
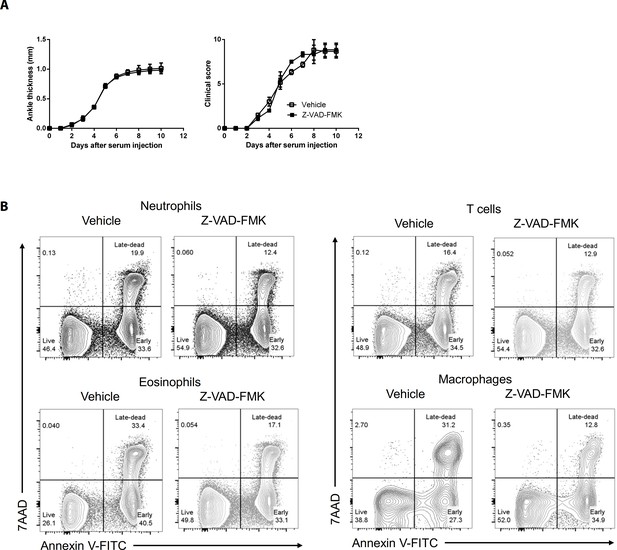
The caspase inhibitor Z–VAD–FMK does not affect AIA in WT mice but induces apoptosis in joint immune cells Joint swelling and clinical scores (A) and apoptosis (B) in joint immune cells were measured in WT mice injected with Z–VAD–FMK or the vehicle only (n = 6 per group).
Data were pooled from three independent experiments and are presented as mean ± SEM.
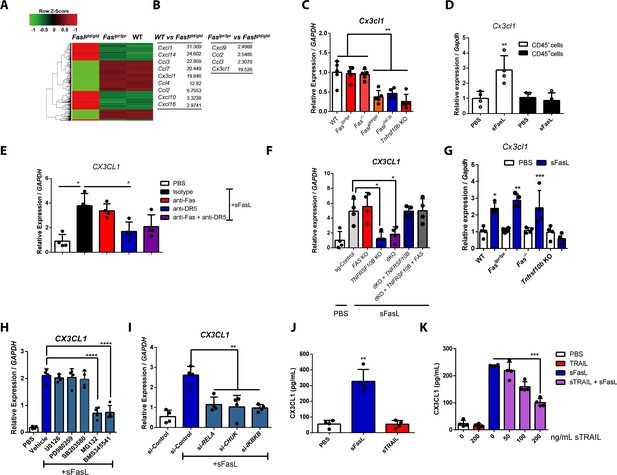
The sFasL–DR5 interaction enhances CX3CL1 expression by human and mouse FLSCs.
(A, B) Microarray assay using joint tissues from WT, Faslpr/lpr, and Faslgld/gld mice with arthritis. (C) Cx3cl1 transcript levels estimated in joint tissues from WT, Faslpr/lpr, Fas–/–, Faslgld/gld, FaslΔs/Δs, and Tnfrsf10b KO mice with arthritis. (D) Cx3cl1 expression in CD45+ immune and CD45– non–immune cells from the joints of WT mice with arthritis after sFasL treatment. (E, F) CX3CL1 transcript levels estimated in hFLSCs in the presence of anti-Fas and/or anti-DR5 antibodies (E) and FAS (Fas), TNFSF10B (DR5), or FAS, and TNFRSF10B DKO, or TNFRSF10B and FAS overexpression in DKO hFLSCs (F). (G) Cx3cl1 expression in synovial fibroblasts from WT, Faslpr/lpr, Fas–/–, or Tnfrsf10b KO mice in the presence or absence of sFasL. (H, I) CX3CL1 transcript levels estimated after sFasL stimulation in hFLSCs in the presence of MEK (U0126), ERK (PD980259), p38 kinase (SB203580), and NF-κB (MG132 and BMS345541) inhibitors (H) or transfection with control, RELA, CHUK (IKKa), or IKBKB (IKKb) siRNA (I). (J) Synovial fibroblasts obtained from WT mice with arthritis were incubated with sFasL or sTRAIL and CX3CL1 levels were measured using ELISA. (K) hFLSCs were stimulated with sFasL after preincubation with various concentrations of sTRAIL for 30 min and CX3CL1 levels were measured in the culture supernatant. Data were pooled from three (C–G and K) or four (H–J) independent experiments and are presented as mean ± SEM (n = 4 for C–K). *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
-
Figure 3—source data 1
Numerical data obtained during experiments represented in Figure 3, Figure 3—figure supplements 1 and 2.
- https://cdn.elifesciences.org/articles/48840/elife-48840-fig3-data1-v1.xlsx
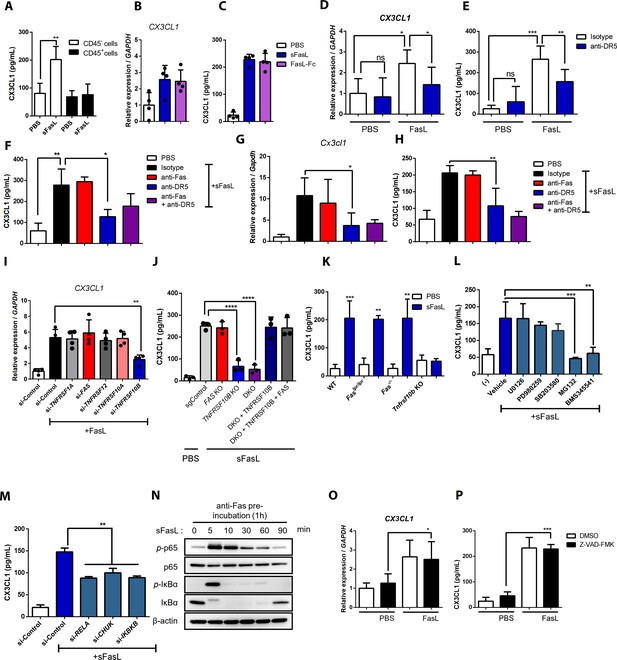
CX3CL1 expression and NF-κB signaling in FasL-treated hFLSCs and mouse synovial fibroblasts.
(A) CX3CL1 was measured in culture supernatants of CD45+ immune and CD45– non-immune cells from the joints of WT mice with arthritis after sFasL stimulation. (B–H) Cx3cl1 transcripts and CX3CL1 protein in culture supernatants were estimated in human (B–F) and mouse (G, H) FLSCs after stimulation with sFasL or FasL–Fc in the presence of anti-Fas or anti-DR5 antibodies. (I) CX3CL1 transcript of hFLSCs transfected with control, TNFRSF1A, FAS, TNFRSF12, TNFRSF10A, and TNFRSF10B siRNA was measured after stimulation with sFasL. (J) FAS and/or TNFRSF10B KO hFLSCs, and DKO hFLSCs were transfected with TNFRSF10B. FAS in the expression vector was stimulated with sFasL. CX3CL1 levels were measured in the culture supernatants. (K) Levels of CX3CL1 in culture supernatants of synovial fibroblasts obtained from WT, Faslpr/lpr, Fas–/–, or Tnfrsf10b KO mice with arthritis after incubation with sFasL. (L, M) Levels of CX3CL1 in culture supernatants of hFLSCs after sFasL stimulation for 2 hr in the presence of MEK (U0126), ERK (PD980259), p38 kinase (SB203580), and NF-κB (MG132, and BMS345541) inhibitors (L) or transfection with control, RELA, CHUK (IKKa), or IKBKB (IKKb) siRNA (M). (N) Blotting assay for components of the NF-κB signaling pathway in hFLSCs stimulated with sFasL for the durations indicated, all preincubated with anti-Fas antibodies. (O, P) CX3CL1 transcript (O) and CX3CL1 protein (P) levels in culture supernatants from hFLSCs after stimulation with sFasL in the presence of 50 μM Z–VAD–FMK. Data were pooled from four (A–F, L, and M) or three (G–K, O, and P) independent experiments and are presented as mean ± SEM (n = 5; A–M, O, and P). NS, not significant; *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
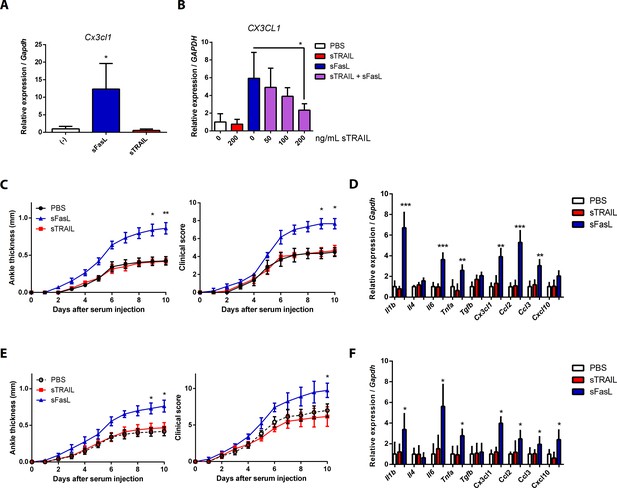
CX3CL1 transcript expression pattern in human and mouse synovial fibroblasts, and arthritis in Faslgld/gld and WT mice upon sFasL and sTRAIL treatment.
(A) Synovial fibroblasts obtained from WT mice with arthritis were incubated with sFasL or sTRAIL. Cx3cl1 transcripts were measured (n = 4). (B) hFLSCs were stimulated with sFasL after preincubation with indicated concentrations of sTRAIL for 30 min and CX3CL1 transcripts were measured (n = 4). (C–F) Joint swelling and clinical scores, as well as transcript levels of various cytokines and chemokines in joint tissues from Faslgld/gld (C, D) and WT (E, F) mice injected with sFasL or sTRAIL (n = 6 per group). Data were pooled from three independent experiments and are presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
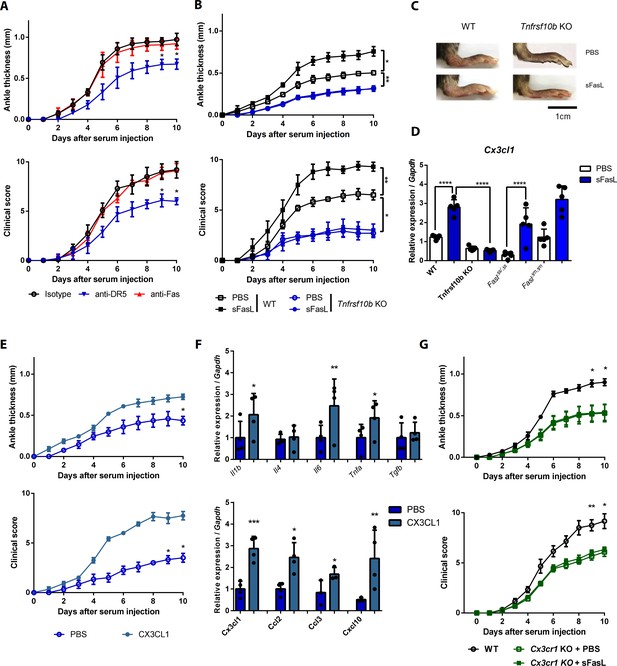
The sFasL–DR5 interaction promotes joint inflammation via the CX3CL1–CX3CR1 axis.
(A) Joint swelling and clinical scores in WT mice injected with anti-DR5 or anti-Fas antibodies to measure AIA (n = 5 per group). (B, C) Joint swelling and clinical scores in WT and Tnfrsf10b KO mice injected with sFasL or phosphate-buffered saline (PBS) to measure AIA (n = 5 per group). (D) Cx3cl1 transcript levels in the joints were estimated in WT, Tnfrsf10b KO, FaslΔs/Δs, and FaslΔm/Δm mice injected with sFasL or PBS to measure AIA (n = 5). (E, F) Joint swelling and clinical scores (E), and transcript levels of various cytokines and chemokines in joint tissues of Tnfrsf10b KO mice injected with CX3CL1 or PBS to measure AIA (F) (n = 6 per group). (G) Joint swelling and clinical scores of WT and Cx3cr1 KO mice in the presence or absence of sFasL to measure AIA (n = 6 per group). Data were pooled from three independent experiments and are presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
-
Figure 4—source data 1
Numerical data obtained during experiments represented in Figure 4, Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/48840/elife-48840-fig4-data1-v1.xlsx

Measurement of arthritis related to Figure 4.
(A, B) Joint swelling and clinical scores together with transcript levels of various cytokines and chemokines in joint tissues from Faslgld/gld mice injected with sFasL, as well as anti-Fas, or anti-DR5 antibodies (n = 6 per group). (C, D) Joint swelling and clinical scores in FaslΔs/Δs (C) and Faslgld/gld (D) mice injected with CX3CL1 or PBS. (E, F) Transcript levels of various cytokines and chemokines in joint tissues from FaslΔs/Δs (E) or Faslgld/gld (F) mice injected with CX3CL1 or PBS to measure AIA (n = 6 per group). Data were pooled from four (A, B) or three (C–F) independent experiments and are presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
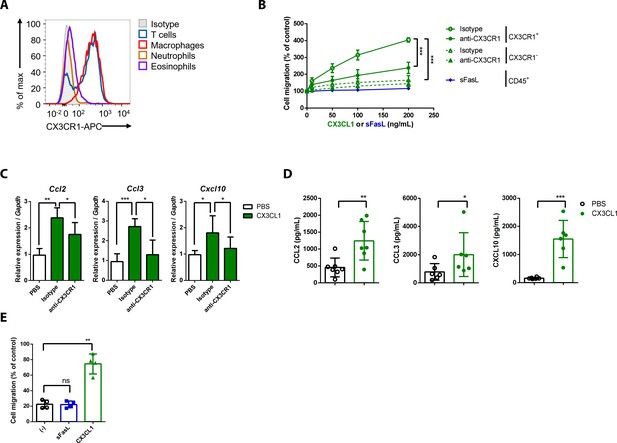
CX3CL1 stimulation increases the migration and production of chemokines in human and mouse CX3CR1+ cells.
(A) Flow cytometric analysis of CX3CR1 expression in immune cells from arthritic joint tissues. (B) Migration assay for synovial CX3CR1+ or CX3CR1– immune cells upon stimulation with CX3CL1 in the presence or absence of anti-CX3CR1 antibodies (green) and CD45+ immune cells stimulated with sFasL (blue; n = 5). (C) Ccl2, Ccl3, and Cxcl10 transcript levels in synovial macrophages after stimulation with CX3CL1 (n = 6). (D) Levels of chemokines in supernatants of synovial leukocyte cultures from patients with rheumatoid arthritis, measured using ELISA after treatment with CX3CL1 (n = 6). (E) Migration assay of synovial leukocytes from patients with rheumatoid arthritis after stimulation with sFasL or CX3CL1 (n = 4). Data were pooled from three (A, C, and D) or four (B, E) independent experiments and are presented as mean ± SEM of independent experiments. *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
-
Figure 5—source data 1
Numerical data obtained during experiments represented in Figure 5.
- https://cdn.elifesciences.org/articles/48840/elife-48840-fig5-data1-v1.xlsx

Gating strategy for flow cytometry analyses of CX3CR1 expression in immune cells from arthritic joint tissues.
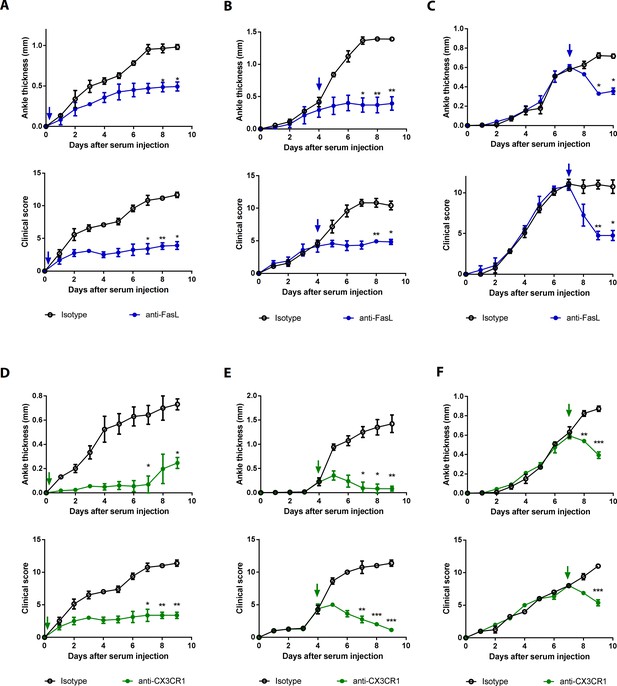
Blockade of FasL or CX3CR1 at different phases of AIA-attenuated joint inflammation.
(A–F) Ankle thicknesses of WT mice injected with anti-FasL or anti-CX3CR1 antibodies (10 μg per mouse daily) on days 0–9 (A, D), days 5–9 (B, E), and days 8–10 (C, F) to measure AIA (A–C, n = 6; D–F, n = 4). The arrows in the diagrams indicate the day on which antibodies were first injected. Data were pooled from three independent experiments and are presented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.005. Data were analyzed using one-way ANOVA.
-
Figure 6—source data 1
Numerical data obtained during experiments represented in Figure 6, Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/48840/elife-48840-fig6-data1-v1.xlsx
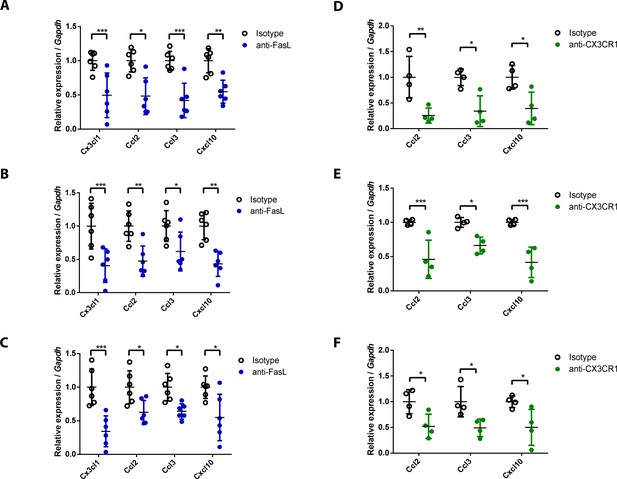
Chemokine expression levels in joint tissues from WT mice injected with anti-FasL or anti-CX3CR1 antibodies at various time points.
(A–F) Expression levels of chemokines in the joint tissues of WT mice injected with 10 μg anti-FasL or anti-CX3CR1 antibodies per day on days 0–9 (A, D), days 5–9 (B, E), or days 8–10 (C, F) to measure AIA (A–C, n = 6; D–F, n = 4). Data were pooled from three independent experiments and are presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.005. Data were analyzed using one-way ANOVA.
Tables
The list of proteins obtained from AP-MS experiment.
| Gene | Description | Location | Family |
|---|---|---|---|
| JUP | Junction plakoglobin | Plasma Membrane | Other |
| HBA1/HBA2 | Hemoglobin, alpha 1 | Extracellular Space | Transporter |
| FASLG | Fas ligand (TNF superfamily, member 6) | Extracellular Space | Cytokine |
| AFP | Alpha-fetoprotein | Extracellular Space | Transporter |
| PKP1 | Plakophilin 1 | Plasma Membrane | Other |
| TNFRSF10B | Tumor necrosis factor receptor superfamily, member 10b | Plasma Membrane | Transmembrane receptor |
| LAMA3 | Laminin, alpha 3 | Extracellular Space | Other |
| EPHA4 | EPH receptor A4 | Plasma Membrane | Kinase |
| LTF | Lactotransferrin | Extracellular Space | Peptidase |
| ABCB1 | ATP-binding cassette, sub-family B (MDR/TAP), member 1 | Plasma Membrane | Transporter |
| LRP2 | Low-density lipoprotein receptor-related protein 2 | Plasma Membrane | Transporter |
| RIMS1 | Regulating synaptic membrane exocytosis 1 | Plasma Membrane | Other |
| PTPRD | Protein tyrosine phosphatase, receptor type, D | Plasma Membrane | Phosphatase |
| ATRN | Attractin | Extracellular Space | Other |
| ADAM30 | ADAM metallopeptidase domain 30 | Plasma Membrane | Peptidase |
| HLA-B* | Major histocompatibility complex, class I, B | Plasma Membrane | Transmembrane receptor |
| KCNC2 | Potassium voltage-gated channel, Shaw-related subfamily, member 2 | Plasma Membrane | Ion channel |
| SPPL2A | Signal peptide peptidase like 2A | Plasma Membrane | Peptidase |
| BSN | Bassoon presynaptic cytomatrix protein | Plasma Membrane | Other |
| PTPRG | Protein tyrosine phosphatase, receptor type, G | Plasma Membrane | Phosphatase |
| LRRC23 | Leucine-rich repeat containing 23 | Plasma Membrane | Other |
| LTBP3 | Latent transforming growth factor beta binding protein 3 | Extracellular Space | Other |
| PLA2R1 | Phospholipase A2 receptor 1, 180 kDa | Plasma Membrane | Transmembrane receptor |
| OGFR | Opioid growth factor receptor | Plasma Membrane | Other |
| MET | MET proto-oncogene, receptor tyrosine kinase | Plasma Membrane | Kinase |
| NLGN2 | Neuroligin 2 | Plasma Membrane | Enzyme |
| CD70 | CD70 molecule | Extracellular Space | Cytokine |
| HLA-A* | Major histocompatibility complex, class I, A | Plasma Membrane | Other |
| SPTBN1* | Spectrin, beta, non-erythrocytic 1 | Plasma Membrane | Other |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | anti-DR5 antibody (Polyclonal) | Abcam | Abcam:ab-8416; RRID:AB_306551 | Immunohistochemistry(1:50, v/v) |
| Antibody | PE-conjugated Annexin V | BD Biosciences | BD:556421 | Flow cytometry(1:50, v/v) |
| Antibody | Alexa700-conjugated anti-mouse CX3CR1(SA011F11) | Biolegend | Biolegend:149035; RRID:AB_2629605 | Flow cytometry(1:200, v/v) |
| Antibody | APC-conjugated anti-human Fas(DX2) | Biolegend | Biolegend:305612; RRID:AB_314550 | Flow cytometry(1:200, v/v) |
| Antibody | APC-conjugated anti-mouse CX3CR1(SA011F11) | Biolegend | Biolegend:149007; RRID:AB_2564491 | MACS-sorting (1:50, v/v) |
| Antibody | APC-conjugated anti-mouse F4/80(BM8) | Biolegend | Biolegend:123115; RRID:AB_893493 | Flow cytometry(1:200, v/v) |
| Antibody | BV421-conjungated anti-human FasL(NOK-1) | Biolegend | Biolegend:306411; RRID:AB_2716104 | Flow cytometry(1:200, v/v) |
| Antibody | FITC-conjugated anti-human IgG Fc (HP6017) | Biolegend | Biolegend:409309; RRID:AB_2561854 | Flow cytometry(1:200, v/v) |
| Antibody | FITC-conjugated anti-mouse CD11b(M1/70) | Biolegend | Biolegend:101205; RRID:AB_312788 | Flow cytometry(1:200, v/v) |
| Antibody | PE-conjugated anti-human DR5(DJR2-4) | Biolegend | Biolegend:307405; RRID:AB_314677 | Flow cytometry(1:200, v/v) |
| Antibody | PE-conjugated anti-human IgG Fc (HP6017) | Biolegend | Biolegend:409303; RRID:AB_10900424 | Flow cytometry(1:200, v/v) |
| Antibody | PE-conjugated anti-mouse DR5(MD5-1) | Biolegend | Biolegend:119905; RRID:AB_345401 | Flow cytometry(1:200, v/v) |
| Antibody | PE-Cy7-conjugated anti-mouse Ly6G(1A8) | Biolegend | Biolegend:127617; RRID:AB_1877262 | Flow cytometry(1:200, v/v) |
| Antibody | PerCP-Cy5.5-conjugated anti-mouse CD45(30-F11) | Biolegend | Biolegend:103131; RRID:AB_893344 | Flow cytometry(1:200, v/v) |
| Antibody | Ultra-LEAF Purified anti-mouse CX3CR1 Recombinant antibody(QA16A03) | Biolegend | Biolegend:153707, RRID:AB_2721771 | In vitro treatment(10 μg/mL) |
| Antibody | DR5 (D4E9) XP Rabbit mAb(D4E9) | Cell signaling | Cell signaling:8074T; RRID:AB_10950817 | Western blot, Co-IP(1:1000, v/v, 1:50, v/v(Co-IP)) |
| Antibody | Fas (C18C12) Rabbit mAb(C18C12) | Cell signaling | Cell signaling:4233T; RRID:AB_2100359 | Western blot(1:1000, v/v) |
| Antibody | FasL (D1N5E) Rabbit mAb(D1N5E) | Cell signaling | Cell signaling:68405S; RRID:AB_2799745 | Western blot(1:1000, v/v) |
| Antibody | His-Tag (D3I1O) XP Rabbit mAb(D3I1O) | Cell signaling | Cell signaling:12698S; RRID:AB_2744546 | Co-IP (1:1000, v/v) |
| Antibody | IκBα (L35A5) Mouse mAb | Cell signaling | Cell signaling:4814; RRID:AB_390781 | Western blot (1:1000, v/v) |
| Antibody | NF-κB p65 (D14E12) XP Rabbit mAb | Cell signaling | Cell signaling:8242; RRID:AB_10859369 | Western blot (1:1000, v/v) |
| Antibody | Phospho-IκBα (Ser32) (14D4) Rabbit mAb | Cell signaling | Cell signaling:2859; RRID:AB_561111 | Western blot (1:1000, v/v) |
| Antibody | Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb | Cell signaling | Cell signaling:3033; RRID:AB_331284 | Western blot (1:1000, v/v) |
| Antibody | TRAIL (C92B9) Rabbit mAb (C92B9) | Cell signaling | Cell signaling:3219S; RRID:AB_2205818 | Western blot (1:1000, v/v) |
| Antibody | β-Actin (13E5) Rabbit mAb | Cell signaling | Cell signaling:4970; RRID:AB_2223172 | Western blot (1:1000, v/v) |
| Antibody | Anti-Mouse CD178 (Fas Ligand)(MFL3) | eBioscience | eBioscience:16-5911; RRID:AB_469145 | In vivo injection (50 μg / injection) |
| Antibody | Anti-Fas Antibody (human, neutralizing)(ZB4) | Merck | Merck:05-338; RRID:AB_309682 | In vitro treatment (20 μg/mL) |
| Antibody | Human Fas Ligand/TNFSF6 Antibody(100419) | R&D | R&D:MAB126; RRID:AB_2246667 | Neutralization (10 μg/mL) |
| Antibody | Human TRAIL R2/TNFRSF10B Antibody(Polyclonal) | R&D | R&D:AF631; RRID:AB_355489 | Neutralization (20 μg/mL) |
| Antibody | Human/Mouse CX3CR1 Antibody (Polyclonal) | R&D | R&D:AF5825; RRID:AB_2292441 | In vivo injection (10 μg / injection) |
| Antibody | Mouse Fas/TNFRSF6/CD95 Antibody (Polyclonal) | R&D | R&D:AF435; RRID:AB_355358 | In vivo injection (50 μg / injection) |
| Antibody | Mouse IgG2B Isotype Control(73009) | R&D | R&D:MAB0042; RRID:AB_471245 | Control (10 μg/mL) |
| Antibody | Mouse TRAIL R2/TNFRSF10B Antibody(Polyclonal) | R&D | R&D:AF721; RRID:AB_205069 | In vivo injection (50 μg / injection) |
| Antibody | Normal Goat IgG Control (Polyclonal) | R&D | R&D:AB-108-C; RRID:AB_354267 | Control (20 μg/mL) |
| Antibody | Normal Goat IgG Control (Polyclonal) | R&D | R&D:AB-108-C; RRID:AB_354267 | In vivo injection (50 μg / injection) |
| Biological sample (H. sapiens) | Human fibroblast-like synoviocytes (hFLSCs) | PMID:19709444 | See materials and methods | |
| Cell line (M. musculus) | EL4 | Korean Cell Line Bank | KCLB:40039 | |
| Cell line (M. musculus) | Jurkat | Korean Cell Line Bank | KCLB:40152 | |
| Chemical compound, drug | Z-VAD-FMK | Calbiochem | Calbiochem:187389-52-2 | 50 μM |
| Chemical compound, drug | Z-VAD-FMK | Calbiochem | Calbiochem:187389-52-2 | 50 μg / mouse |
| Chemical compound, drug | Protein A/G PLUS-agarose beads | Santa Cruz Biotechnology | Sanat cruz:sc-2003 | |
| Chemical compound, drug | BMS345541 | Sigma | Sigma:B9935 | 10 mM |
| Chemical compound, drug | MG132 | Sigma | Sigma:M7449 | 10 mM |
| Chemical compound, drug | NSCI | Sigma | Sigma:N1413 | 5 μM |
| Chemical compound, drug | PD980259 | Sigma | Sigma:513000 | 10 mM |
| Chemical compound, drug | SB203580 | Sigma | Sigma:S8307 | 10 mM |
| Chemical compound, drug | U0126 | Sigma | Sigma:U120 | 10 mM |
| Chemical compound, drug | BS3 (bis-(sulfo-succinimidyl) suberate) | Thermo Fisher Scientific | Thermo Fisher:21580 | See materials and methods |
| Chemical compound, drug | EZ Link Sulfo-NHS-SS-Biotin | Thermo Fisher Scientific | Thermo Fisher:A39258 | Biotinylation of Fas ligand (10mM) |
| Commercial assay or kit | Anti-APC microbeads | Miltenyi Biotec | Miltenyi Biotec:130-090-855 | Cell isolation (1:50, v/v) |
| Commercial assay or kit | RNeasy Mini kit | Qiagen | Qiagen:74104 | |
| Commercial assay or kit | Human CCL2/MCP-1 Quantikine ELISA Kit | R&D | R&D:SCP00 | |
| Commercial assay or kit | Human CCL3/MIP-1 alpha Quantikine ELISA Kit | R&D | R&D:SMA00 | |
| Commercial assay or kit | Human CX3CL1/Fractalkine DuoSet ELISA | R&D | R&D:DY365 | |
| Commercial assay or kit | Human CXCL10/IP-10 Quantikine ELISA Kit | R&D | R&D:SIP100 | |
| Commercial assay or kit | Mouse CX3CL1/Fractalkine DuoSet ELISA | R&D | R&D:DY472 | |
| Commercial assay or kit | Mouse Fas Ligand/TNFSF6 Quantikine ELISA Kit | R&D | R&D;MFL00 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Ccl2) | Thermo Fisher Scientific | Thermo Fisher:Mm00441242_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Ccl3) | Thermo Fisher Scientific | Thermo Fisher:Mm00441259_g1 | |
| Genetic reagent (H. sapiens) | Taqman gene expression assay (CX3CL1) | Thermo Fisher Scientific | Thermo Fisher:Hs00171086_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Cx3cl1) | Thermo Fisher Scientific | Thermo Fisher:Mm00436454_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Cxcl10) | Thermo Fisher Scientific | Thermo Fisher:Mm00445235_m1 | |
| Genetic reagent (H. sapiens) | Taqman gene expression assay (GAPDH) | Thermo Fisher Scientific | Thermo Fisher:Hs02786624_g1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Gapdh) | Thermo Fisher Scientific | Thermo Fisher:Mm99999915_g1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Il1b) | Thermo Fisher Scientific | Thermo Fisher:Mm00434228_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Il4) | Thermo Fisher Scientific | Thermo Fisher:Mm00445259_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Il6) | Thermo Fisher Scientific | Thermo Fisher:Mm00446190_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Tgfb) | Thermo Fisher Scientific | Thermo Fisher:Mm01178820_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Tnfa) | Thermo Fisher Scientific | Thermo Fisher:Mm00443258_m1 | |
| Genetic reagent (H. sapiens) | Taqman gene expression assay (TNFRSF10B) | Thermo Fisher Scientific | Thermo Fisher:Hs00366278_m1 | |
| Genetic reagent (H. sapiens) | Taqman gene expression assay (TNFRSF1A) | Thermo Fisher Scientific | Thermo Fisher:Hs01042313_m1 | |
| Genetic reagent (H. sapiens) | Taqman gene expression assay (TNFRSF10A) | Thermo Fisher Scientific | Thermo Fisher:Hs00269492_m1 | |
| Genetic reagent (H. sapiens) | Taqman gene expression assay (TNFRSF12) | Thermo Fisher Scientific | Thermo Fisher:Hs00171993_m1 | |
| Genetic reagent (M. musculus) | Taqman gene expression assay (Tnfrsf10b) | Thermo Fisher Scientific | Thermo Fisher:Mm00457866_m1 | |
| Genetic reagent (H. sapiens) | TrueGuide sgRNA (FAS) | Thermo Fisher Scientific | Thermo Fisher:CRISPR872691_SGM | 7.5 pmol |
| Genetic reagent (H. sapiens) | TrueGuide sgRNA (negative control) | Thermo Fisher Scientific | Thermo Fisher:A35526 | 7.5 pmol |
| Genetic reagent (H. sapiens) | TrueGuide sgRNA (TNFRSF10B) | Thermo Fisher Scientific | Thermo Fisher:CRISPR606464_SGM | 7.5 pmol |
| Other | 7-AAD | BD Biosciences | BD:559925 | Flow cytometry(1:50, v/v) |
| Other | Propidium Iodide Staining Solution | BD Biosciences | BD:556463 | Flow cytometry(1:50, v/v) |
| Peptide, recombinant protein | Recombinant Human Fas Ligand/TNFSF6 Protein | R&D | R&D:126-FL | In vitro treatment(2 μM) |
| Peptide, recombinant protein | Recombinant Human TRAIL R2/TNFRSF10B Fc Chimera Protein | R&D | R&D:631-T2 | In vitro treatment(200 ng/mL) |
| Peptide, recombinant protein | Recombinant Human TRAIL/TNFSF10 Protein | R&D | R&D:375-TL | Flow cytometry(200 ng/mL) |
| Peptide, recombinant protein | Recombinant Mouse CX3CL1/Fractalkine (Full Length) Protein | R&D | R&D:472-FF | In vitro treatment(200 ng/mL) |
| Peptide, recombinant protein | Recombinant Mouse CX3CL1/Fractalkine (Full Length) Protein | R&D | R&D:472-FF | In vivo injection (1 μg/injection) |
| Peptide, recombinant protein | Recombinant Mouse Fas Ligand/TNFSF6 Protein | R&D | R&D:526-SA | In vivo injection(1 μg/mouse) |
| Peptide, recombinant protein | Recombinant Mouse Fas Ligand/TNFSF6 Protein | R&D | R&D:526-SA | In vitro treatment(200 ng/mL) |
| Peptide, recombinant protein | Recombinant Mouse TRAIL | R&D | R&D:1121-TL | In vitro treatment (200 ng/mL) |
| Peptide, recombinant protein | Recombinant Mouse TRAIL | R&D | R&D:1121-TL | In vivo injection (1 μg/mouse) |
| Peptide, recombinant protein | Truecut Cas9 protein | Thermo Fisher Scientific | Thermo Fisehr:A36498 | 7.5 pmol |
| Peptide, recombinant protein | FasL-Fc | Y-Biologics | This paper | See materials and methods |
| Peptide, recombinant protein | Recombinant Human TNF-alpha | R&D | R&D:210-TA | See materials and methods |
| Peptide, recombinant protein | Recombinant Mouse TNF-alpha | R&D | R&D:410-MT | See materials and methods |
| Recombinant DNA reagent | pIRESpuro3 | Clontech | Clontech:631619 | See materials and methods |
| Sequence-based reagent | MISSION siRNA (CUHK) | Sigma | Sigma:SASI_Hs01_00206921 | |
| Sequence-based reagent | MISSION siRNA (FAS) | Sigma | Sigma:SASI_Hs02_00301734 | |
| Sequence-based reagent | MISSION siRNA (IKBKB) | Sigma | Sigma:SASI_Hs01_00156170 | |
| Sequence-based reagent | MISSION siRNA (RELA) | Sigma | Sigma:SASI_Hs01_00171091 | |
| Sequence-based reagent | MISSION siRNA (TNFRSF10A) | Sigma | Sigma:SASI_Hs01_00139573 | |
| Sequence-based reagent | MISSION siRNA (TNFRSF10B) | Sigma | Sigma:SASI_Hs01_00040567 | |
| Sequence-based reagent | MISSION siRNA (TNFRSF12A) | Sigma | Sigma:SASI_Hs01_00129286 | |
| Sequence-based reagent | MISSION siRNA (TNFRSF1A) | Sigma | Sigma:SASI_Hs01_00033456 | |
| Software, algorithm | FlowJo | FlowJo | Version 10 | |
| Software, algorithm | BIA evaluation | GE Healthcare Bio-Sciences | GE:BR-1005-97 | |
| Software, algorithm | Illumina GenomeStudio | Gene Expression Module | Version 1.9.0 | |
| Software, algorithm | GraphPad Prism | GraphPad Software | Version 5.0 | |
| Software, algorithm | Sequest algorithm | N/A | Version 27 | |
| Software, algorithm | SORCERER | Sage-N Research | ||
| Strain, strain background (Mus musculus, C57BL/6J) | Faslgld/gld | Japan SLC | ||
| Strain, strain background (Mus musculus, C57BL/6J) | Faslpr/lpr | Japan SLC | ||
| Strain, strain background (Mus musculus, C57BL/6J) | K/BxN | PMID:8945509 | ||
| Strain, strain background (Mus musculus, C57BL/6J) | Tnfrsf10b KO | MMMRC | MMMRC:030532-MU | |
| Strain, strain background (Mus musculus, C57BL/6J) | FaslΔm/Δm | PMID:19794494 | ||
| Strain, strain background (Mus musculus, C57BL/6J) | FaslΔs/Δs | PMID:19794494 | ||
| Strain, strain background (Mus musculus, C57BL/6J) | Wild type C57BL/6 (WT) | Orient Bio | ||
| Strain, strain background (Mus musculus, C57BL/6J) | Cx3cr1 KO | Taconic | Taconic:4167 | |
| Strain, strain background (Mus musculus, C57BL/6J) | Fas-/- | PMID:7581453 | RIKEN BRC:RBRC01474 |





