Vaccine-induced COVID-19 mimicry syndrome
Figures
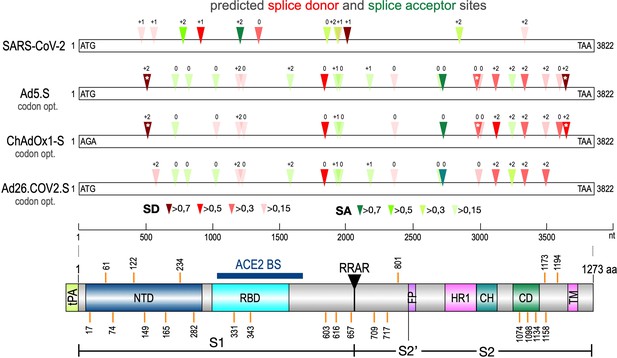
Splice site prediction within the Spike open reading frames.
Splice site prediction was carried out by using SpliceRover. Splice donor sites are given in red, splice acceptor sites in green. SpliceRover calculates splice sites with a score between 0 and 1, but only splice sites with >0.15 were displayed. Sites were displayed in four colors as indicated. Splice sites were numbered with ‘0’, ‘+1’, or ‘+2’, to indicate how the open reading frame is disrupted. Therefore, all splice events between splice sites with identical numbers will be in-frame, while all splice reactions between unequal numbers will result in out-of-frame fusions. Below, the protein domain structure of Spike is displayed to explain, which domains are being deleted by splice events (NTD: N-terminal domain, RBD: (ACE2) receptor-binding domain, RRAR: furin cleavage site, HR1: heptad repeat1, CH: central helix, CD: connector domain, TM: transmembrane domain). White asterisks mark three splice donor sites that are present in the codon-optimized Spike reading frame of the Vaxzevria but not of the Janssen vaccine.
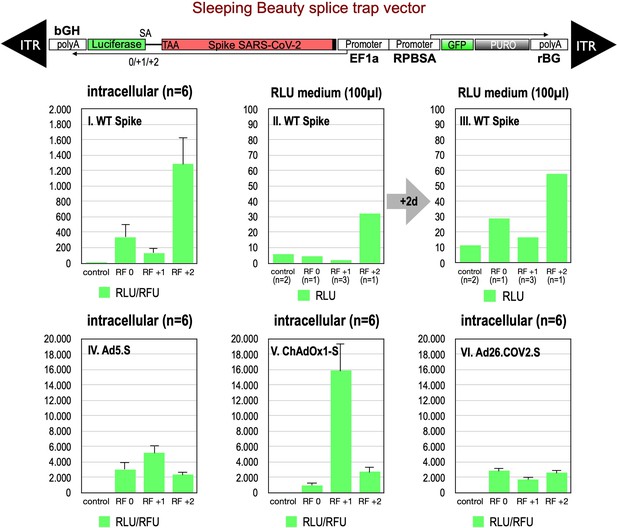
Splice trap Luciferase experiments in HEK293T cells.
The three different splice traps were cloned into the pSBbi-GP vector. This vector encodes two polycistronic transcripts, one encoding GFP and Puromycin resistance (from left to right), while the other encodes the full-length Spike gene connected to an artificial intron and an ATG-deleted Luciferase gene. Splice events to the Luciferase transcript will result in ‘in-frame’ and ‘out-of-frame’ Spike-Luciferase fusion transcripts. HEK293T cells were stably transfected with the constructs and resulting Luciferase activity was measured. RLU: relative light units; RFU: relative fluorescence units. Middle panels I–III: The three different splice trap constructs encode the wildtype Spike gene. Luciferase expression was measured intracellularly and extracellularly 3-day post-transfection. Lower panels IV–VI: The three different splice trap constructs encode the codon-optimized Spike sequences derived from either the adenoviral vector Ad5.S, ChAdOx1-S, or Ad26.COV2.S. Luciferase expression was measured intracellularly.
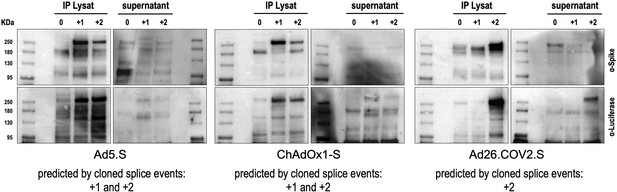
Splice trap Western blot experiments in HEK293T cells.
Spike and Spike-fusion proteins were concentrated with the help of immunoprecipitation beads from all cell lysates and supernatants of HEK293T cells stably transfected with the splice trap constructs encoding the codon-optimized Spike sequences derived from the adenoviral vectors Ad5.S, ChAdOx1-S, or Ad26.COV2.S. Proteins bound to these beads were used to perform Western blot experiments, which are displayed. Bead eluates were separated by SDS-PAGE, blotted, and proteins were detected with anti-Spike or anti-Luciferase antibodies.
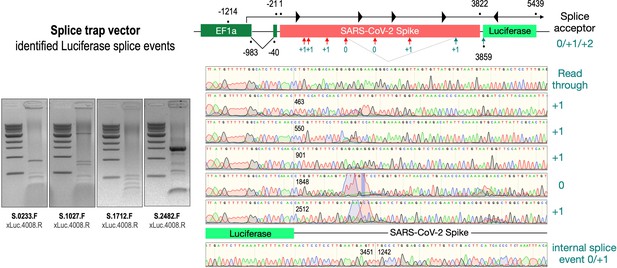
Splice events after transfection of HEK293T cells.
The wildtype SARS-CoV-2 Spike gene – cloned into the three splice trap constructs – was stably transfected in HEK293T cells, RNA was isolated and investigated by RT-PCR experiments for splicing events. Appropriate PCR bands were cut out from the gel and either directly sequenced or cloned for sequencing. The major splice events discovered are displayed.
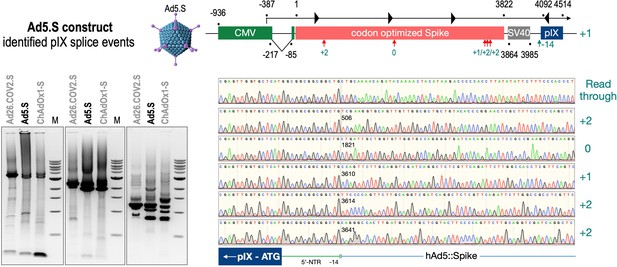
Splice events after Ad5.S transduction of HeLa cells.
HeLa or HepG2 cells were transduced with the adenoviral vectors Ad5.S and RNA was isolated 48 hr post transduction. Three universal primers binding at three different locations within the Spike genes and one universal reverse primer binding to the adenoviral pIX that is located directly adjacent to the Spike gene sequence were used to investigate splicing events. The resulting DNA fragments are shown in the agarose gel on the left. Appropriate PCR bands were excised from the gel and either directly sequenced or cloned for sequencing. The predominately detected splice events are displayed on the right.
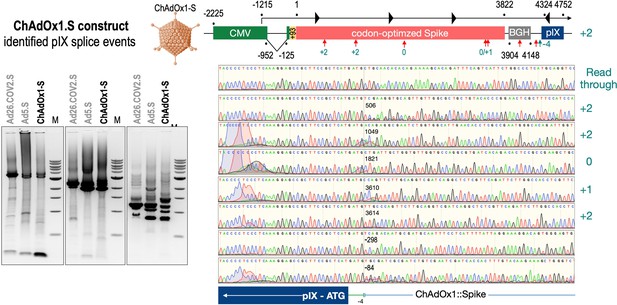
Splice events after ChAdOx1-S transduction of HeLa or HepG2 cells.
HeLa or HepG2 cells were transduced with the adenoviral vector Ad5.S and RNA was isolated 48 hr post transduction. Three universal primers binding at three different locations within the Spike genes and one universal reverse primer binding to the adenoviral pIX that is located directly adjacent to the Spike gene sequence were used to investigate splicing events. The resulting DNA fragments are shown in the agarose gel on the left. Appropriate PCR bands were excised from the gel and either directly sequenced or cloned for sequencing. The predominately detected splice events are displayed on the right.
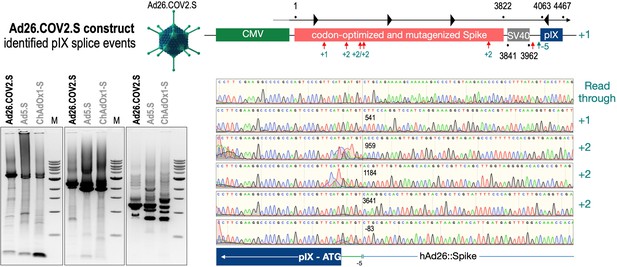
Splice events after Ad26.COV2.S transduction of HeLa or HepG2 cells.
HeLa or HepG2 cells were transduced with the adenoviral vectors Ad5.S and RNA was isolated 48 hr post transduction. Three universal primers binding at three different locations within the Spike genes and one universal reverse primer binding to the adenoviral pIX that is located directly adjacent to the Spike gene sequence were used to investigate splicing events. The resulting DNA fragments are shown in the agarose gel on the left. Appropriate PCR bands were excised from the gel and either directly sequenced or cloned for sequencing. The predominately detected splice events are displayed on the right.
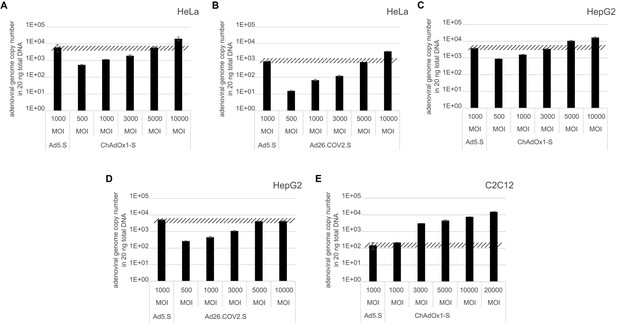
Analysis of required multiplicity of infection (MOI) of different vector strains to achieve equal transduction efficiencies.
HeLa, HepG2, and C2C12 cells were transduced with indicated physical MOI of Ad5.S, ChAdOx1-S, or Ad26.COV2.S. Cells were incubated with the vectors for 2 hr at 37°C and harvested. Total DNA was isolated and intracellular adenoviral genome copy numbers were analyzed by quantitative real-time PCR.
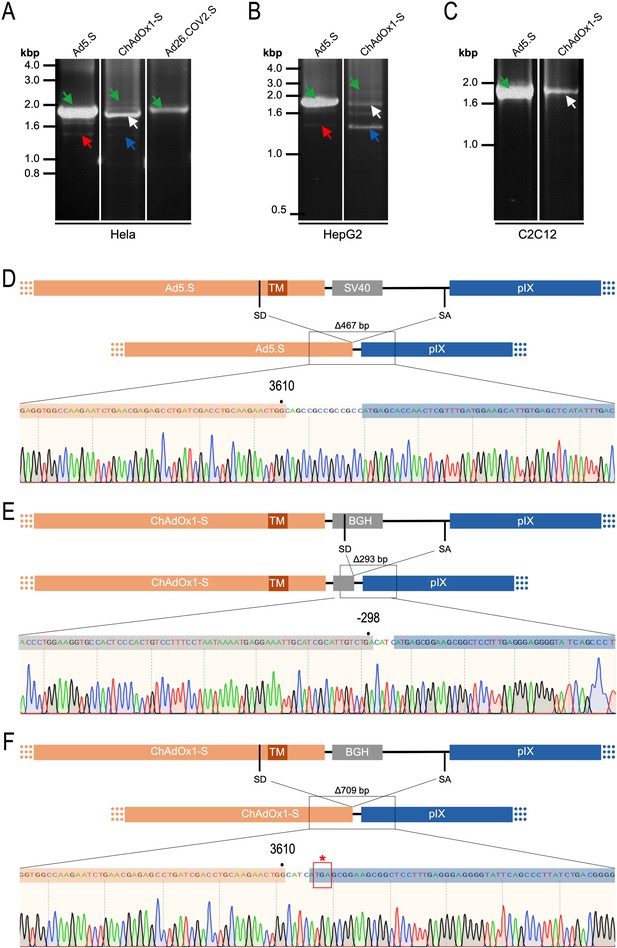
Cell type-dependent and predominant splicing of the ChAdOx1.S Spike sequence.
(A) HeLa, (B) HepG2, and (C) C2C12 cells were transduced with equal efficiencies with the adenoviral vectors Ad5.S, ChAdOx1-S, and Ad26.COV2.S encoding the codon-optimized Spike sequences. Total RNA was extracted 48 hr post transduction. PCRs using forward primers that bind to the Spike sequence and reverse primers that bind to the adjacent downstream pIX in the adenoviral DNA genome were used for PCR amplification of generated cDNAs. Separation of PCR amplicons by agarose gel electrophoresis. Green arrow: Full-length read-through fragments. Red arrow: Spliced fragment of the Ad5.S-encoded Spike sequence resulting in a potentially secreted Spike fusion protein. White arrow: Spliced fragment of the ChAdOx1-S-encoded Spike sequence resulting in a Spike-pIX fusion protein. Blue arrow: Spliced fragment of the ChAdOx1-S-encoded Spike sequence resulting in a potentially secreted Spike protein. (D) Sequencing result of DNA fragment marked with the red arrow. (E) Sequencing result of DNA fragment marked with the white arrow. (F) Sequencing result of DNA fragment marked with the blue arrow.
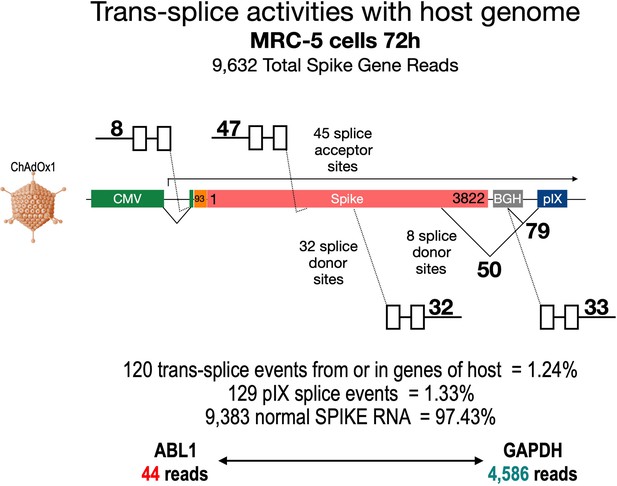
Trans-splicing events of Vaxzevria in MRC-5 cells.
RNA-Seq data were kindly provided by David A Matthews, University of Bristol. RNA-Seq data derived from MRC-5 cells after transduction with the Vaxzevria vaccine. RNA was isolated after 72 hr and sequenced in depths. We used this data set to investigate potential splice events visible in this cell line. Total numbers and locations of detected trans-splice and pIX-splice events within the Spike sequence and the adjacent pIX sequence are graphically depicted. As displayed, out of 9632 Spike reads 120 reads were obtained from trans-splicing events from or to host mRNAs; 129 reads were found that demonstrated splicing upstream of the pIX gene. We also determined the amount of two housekeeping genes (ABL1 and GAPDH) to analyze their read numbers. In comparison to the housekeeping genes, vector-based transcription was quite strong and superseded the amount of GAPDH transcripts.
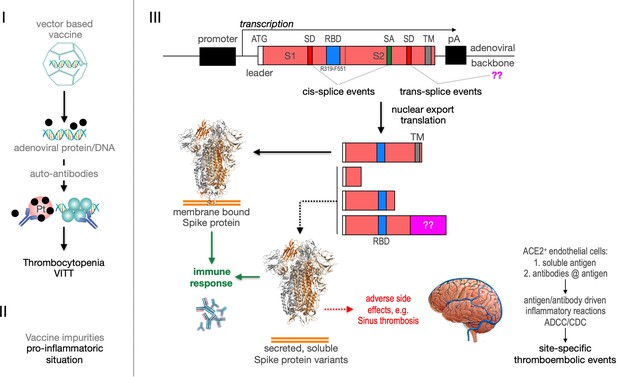
Predicted pathological disease mechanism.
I. Predicts vaccine-induced immune thrombotic thrombocytopenia (VITT) mechanism that is accompanied by the thrombocytopenic situation. II. Pro-inflammatory events which might be caused by vaccine impurities. III. Proposed mechanism that soluble Spike protein variants are generated by distinct splice events. Soluble Spike protein variants which are able to bind to ACE2-expressing endothelial cells may trigger immunological mechanisms that result in thromboembolic events (cerebral venous sinus thromboses [CVST] and splanchnic vein thromboses [SVT]; estimated 1 in 80,000 vaccinated persons in Europe). RBD: receptor-binding domain; TM: transmembrane domain; SD: splice donor; SA: splice acceptor, pA: poly-A sequence;??: fusion to unknown sequences.
Tables
Safety data from the German Paul-Ehrlich Institute (PEI), dated from December 23, 2021.
All data for all four vaccines were directly retrieved from the security report. Data for number of all vaccinations, total cases with complications, anaphyllaxie cases, myo/pericarditis cases, cases with thromboses associated with thrombocytopenia (TTS), cases with thromboses combined with immunothrombocytopenia (ITP), and cases with Guillain-Barré syndrome (GBS) were listed. In addition, for each category the case numbers per 1 million (Mio) injections are given (e.g. the absolute risk for the development of myo/pericarditis after a Biontech vaccination is 12.9 cases per Mio injections). All red numbers differ significantly from the numbers of the other vaccines.
| Company | Biontech/Pfizer | Moderna | Oxford/AZ | Janssen/J&J |
|---|---|---|---|---|
| #Vaccinations | 96.606.131 | 10.576–131 | 12.703.030 | 3.462.557 |
| #Complications | 113.792 | 28.289 | 46.325 | 7.758 |
| Cases per Mio inj. | 1.178 | 2.675 | 3.647 | 2.241 |
| Anaphyllaxie | 550 | 55 | 101 | 10 |
| Cases per Mio inj. | 5,7 | 5.2 | 8.0 | 2,9 |
| Myo/pericarditis | 1245 | 309 | 0 | 0 |
| Cases per Mio inj. | 12.9 | 29.2 | 0 | 0 |
| TTS | 36 | 5 | 200 | 24 |
| Cases per Mio inj. | 0.4 | 0.5 | 15.7 | 6.9 |
| ITP | 314 | 28 | 269 | 23 |
| Cases per Mio inj. | 3.3 | 2.6 | 21.2 | 6.6 |
| GBS | 140 | 14 | 112 | 48 |
| Cases per Mio inj. | 1.4 | 1.3 | 8.8 | 13.9 |
| Death cases | 295 | 20 | 201 | 21 |
| Cases per Mio inj. | 3.1 | 1.9 | 15.8 | 6.1 |
Splice donor site prediction.
The open reading frame of the SARS-CoV-2 Spike protein was analyzed by the Splice Site Predictor online tool SpliceRover (http://bioit2.irc.ugent.be/rover/splicerover). This online tool allows prediction of splice donor and splice acceptor sites. Predicted splice donor sites are provided in Table 1 for the SARS-CoV-2 wildtype Spike gene (A), for the codon-optimized Spike genes derived from the experimental Ad5.S vector, and the FDA/EMA-approved vector-based vaccines from AstraZeneca (Vaxzevria, ChAdOx1-S) and Janssen/J&J (Ad26.COV2.S), respectively. The amino acid coordinates of Spike protein domains S1, S2, and the minimal ACE2-binding domain are indicated.
| Position | Potential splice donor sites | Score | Type |
|---|---|---|---|
| A: Splice donor site prediction in wildtype Spike ORF | |||
| 454–473 | TGGATGGAAA•GTGAGTTCAG | 0.187 | +1 |
| 541–560 | GGAAAACAGG•GTAATTTCAA | 0.151 | +1 |
| 894–911 | GAAACAAAGT•GTACGTTGAA | 0.565 | +1 |
| 1323–1342 | TGATTCTAAG•GTTGGTGGTA | 0.357 | 0 |
| 1906–1925 | TATTCTACAG•GTTCTATTFT | 0.274 | +1 |
| 1996–2015 | ATTGGTGCAG•GTATATGCGC | 0.707 | +1 |
| 3296–3317 | GCACACACTG•GTTTGTAACA | 0.160 | +2 |
| Consensus | MAG•GTNNGTG | ||
| B: Splice donor site prediction in codon-optimized Spike ORF of Ad5 | |||
| 497–516 | GCACCTTCGA•GTACGTGTCC | 0.987 | +2 |
| 1175–1194 | TCACAAACGT•GTACGCCGAC | 0.187 | +2 |
| 1209–1228 | GGGAGATGAA•GTGCGGCAGA | 0.162 | 0 |
| 1812–1831 | CTCCAACCAG•GTGGCCGTGC | 0.540 | 0 |
| 2331–2350 | CACCCAAGAG•GTGTTCGCCC | 0.204 | 0 |
| 2949–2968 | ACTGGACAAG•GTGGAAGCCG | 0.318 | 0 |
| 2961–2980 | GGAAGCCGAG•GTGCAGATCG | 0.151 | 0 |
| 3083–3102 | AGATGTCTGA•GTGTGTGCTG | 0.318 | +2 |
| 3296–3315 | GCACCCATTG•GTTCGTGACC | 0.388 | +2 |
| 3452–3471 | AACTGGATAA•GTACTTTAAG | 0.443 | +2 |
| 3555–3574 | GCTGAACGAG•GTGGCCAAGA | 0.217 | 0 |
| 3605–3624 | AACTGGGGAA•GTACGAGCAG | 0.800 | +2 |
| C: Splice donor site prediction in codon-optimized Spike ORF of ChAdOx1-S | |||
| 497–516 | GCACCTTCGA•GTACGTGTCC | 0.986 | +2 |
| 1012–1030 | TTCGGCGAG• GTGTTCAATG | 0.266 | 0 |
| 1175–1194 | TCACAAACGT•GTACGCCGAC | 0.177 | +2 |
| 1209–1228 | GGGAGATGAA•GTGCGGCAGA | 0.188 | 0 |
| 1812–1831 | CTCCAACCAG•GTGGCCGTGC | 0.541 | 0 |
| 2331–2350 | CACCCAAGAG•GTGTTCGCCC | 0.215 | 0 |
| 2949–2968 | ACTGGACAAG•GTGGAAGCCG | 0.398 | 0 |
| 2961–2980 | GGAAGCCGAG•GTGCAGATCG | 0.273 | 0 |
| 3083–3102 | AGATGTCTGA•GTGTGTGCTG | 0.503 | +2 |
| 3296–3315 | GCACCCATTG•GTTCGTGACC | 0.381 | +2 |
| 3452–3471 | AACTGGATAA•GTACTTTAAG | 0.245 | +2 |
| 3555–3574 | GCTGAACGAG•GTGGCCAAGA | 0.310 | 0 |
| 3605–3624 | AACTGGGGAA•GTACGAGCAG | 0.686 | +2 |
| D: Splice donor site prediction in codon-optimized Spike ORF of Ad26.COV2.S | |||
| 563–582 | ACCTGCGCGA•GTTCGTGTTC | 0.150 | +2 |
| 1175–1194 | TCACAAACGT•GTACGCCGAC | 0.180 | +2 |
| 1209–1228 | GGGAGATGAA•GTGCGGCAGA | 0.207 | 0 |
| 1812–1831 | CAGCAATCAG•GTGGCAGTGC | 0.547 | 0 |
| 2331–2350 | CACCCAAGAG•GTGTTCGCCC | 0.202 | 0 |
| 2961–2980 | TGAGGCCGAG•GTGCAGATCG | 0.194 | 0 |
| 3083–3102 | AGATGTCTGA•GTGTGTGCTG | 0.495 | +2 |
| 3296–3315 | GCACCCATTG•GTTCGTGACA | 0.392 | +2 |
| 3452–3471 | AACTGGACAA•GTACTTTAAG | 0.436 | +2 |
| S1 ectodomain: encoded by nucleotides 1–2049 (aa 1–683) | |||
| S2 domain: encoded by nucleotides 2050–3822 (aa 684–1273) | |||
| ACE2-binding domain: encoded by nucleotides 998–1720 (aa 319–551) | |||
Comparison of all three adenoviral vector-based constructs.
Based on our analysis of three different adenoviral vectors, there were differences between the vaccines. The displayed table analyzes the use of promoter, the different poly(A) elements, and changes inside the sequences of Spike, for example, additional sequences, presence or deletion of the furin cleavage sequence, the addition of two prolines to stabilize of the prefusion configuration of Spike, and site-directed mutations that should avoid splicing.
| Name | Vector | Promotor | Additional sequences | Terminator | RRAR | PP | Splicing |
|---|---|---|---|---|---|---|---|
| Ad5.S | HAd5 | CMV with Intron | – | SV40 | Present | No | + |
| ChAdOx1-S | ChAd | CMV with Intron | + tPA (31aa) | BGH | Present | No | + |
| Ad26.COV2.S | HAd26 | CMV w/o Intron | – | SV40 | Deleted | Present | Splice sites partially deleted |
| Comment | Upstream ATG | pos 682–685 | pos 986/987 | AZ >> J |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/74974/elife-74974-transrepform1-v2.docx
-
Source data 1
Source original data 1.
- https://cdn.elifesciences.org/articles/74974/elife-74974-data1-v2.docx





