Researchers have discovered how a molecule can help prevent certain types of brain tumors by recognizing and ‘disarming’ harmful proteins that cause them.
Their study in fruit flies, published in the journal eLife, could lead to a potential new treatment approach for brain tumors in future.
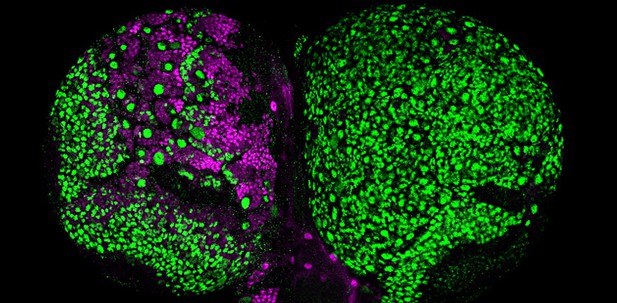
A tumorous brain in fruit flies caused by retromer inactivation (right) as compared to the normal fly brain (left). Li, Wong et al., eLife, 2018.
Most multicellular organisms possess a one-way cell signaling pathway called Notch signaling, which is crucial for embryonic development. The correct establishment and maintenance of Notch signaling are critical for ensuring a balance in the number of stem cells that occur in the body and brain.
“Abnormal activation of Notch signaling in neural progenitors – which send signals to neural stem cells – can cause an excess of these stem cells to occur in the brain, and this can in turn lead to brain tumor development,” explains co-first author Bo Li, PhD Candidate at Peking University, China. “However, the molecular mechanisms that prevent abnormal Notch signaling activation and potentially harmful decisions related to cell fate remain unclear.”
To address this question, the researchers carried out genetic and biochemical tests to study stem cells called neuroblasts in the central brain region of fruit fly larvae. They looked in particular at the retromer protein complex, which transports specific cargo proteins from endosomes (a type of membrane-bound compartment inside cells) to the cell surface.
Their analysis revealed that the retromer complex regulates Notch protein trafficking in neural progenitors. When the complex is inactive, Notch receptors are activated incorrectly, transforming the progenitors into an excess number of neural stem cells and increasing the risk of brain tumor formation.
“We found that a sufficient amount of Notch protein needs to be destroyed in neural progenitors to maintain the one-way Notch signaling pathway between these progenitors and neural stem cells,” explains co-first author Chouin Wong, Graduate Student at Peking University.
“When an excess of Notch protein fails to be destroyed in neural progenitors, this is normally recognized by the retromer complex and is rapidly transported away from the endosomes inside cells. But when the retromer complex is inactive, this pool of Notch protein increases massively in the endosomes and is ‘ignited’ abnormally.”
These results led the team to propose a model whereby the retromer complex serves as a kind of ‘bomb squad’ to retrieve and disarm the potentially harmful pool of incorrectly activated Notch receptors in a timely manner, thereby preventing the risk of brain tumors forming in this way.
“In light of these findings, we believe further investigation into the regulatory mechanisms underlying Notch overactivation is necessary,” concludes senior author Yan Song, Principal Investigator at Peking University. “In particular, a deeper understanding of how to prevent abnormal Notch signaling activation in neural progenitors could potentially provide a new approach for treating brain tumors in future.”
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373
About
eLife aims to help scientists accelerate discovery by operating a platform for research communication that encourages and recognises the most responsible behaviours in science. We publish important research in all areas of the life and biomedical sciences, including Neuroscience, which is selected and evaluated by working scientists and made freely available online without delay. eLife also invests in innovation through open source tool development to accelerate research communication and discovery. Our work is guided by the communities we serve. eLife is supported by the Howard Hughes Medical Institute, the Max Planck Society, the Wellcome Trust and the Knut and Alice Wallenberg Foundation. Learn more at https://elifesciences.org.
To read the latest Neuroscience research published in eLife, please visit https://elifesciences.org/subjects/neuroscience.




