A novel GTP-binding protein–adaptor protein complex responsible for export of Vangl2 from the trans Golgi network
Figures
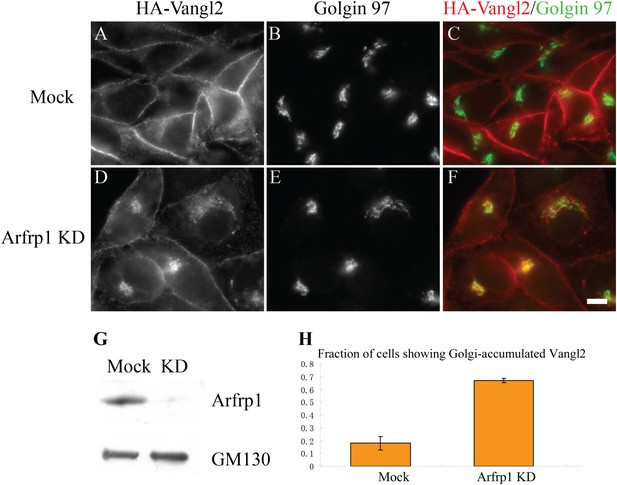
Knockdown of Arfrp1 leads to accumulation of Vangl2 at the TGN.
(A)–(F) HeLa cells stably expressing HA-Vangl2 were either mock transfected or transfected with siRNA against Arfrp1. At day 3 after transfection, the cells were analyzed by indirect immunofluorescence. Size bar = 10 μM. (G) HeLa cell lysates from cells transfected with control siRNA or siRNA against Arfrp1 were analyzed by immunoblotting with anti-Arfrp1 antibody and, as a loading control, anti-GM130 antibody. (H) Quantification of the fraction of cells showing Golgi-accumulated Vangl2 in control or siRNA-treated HeLa cells stably expressing HA-Vangl2 (N = 3; >100 cells counted for each experiment).
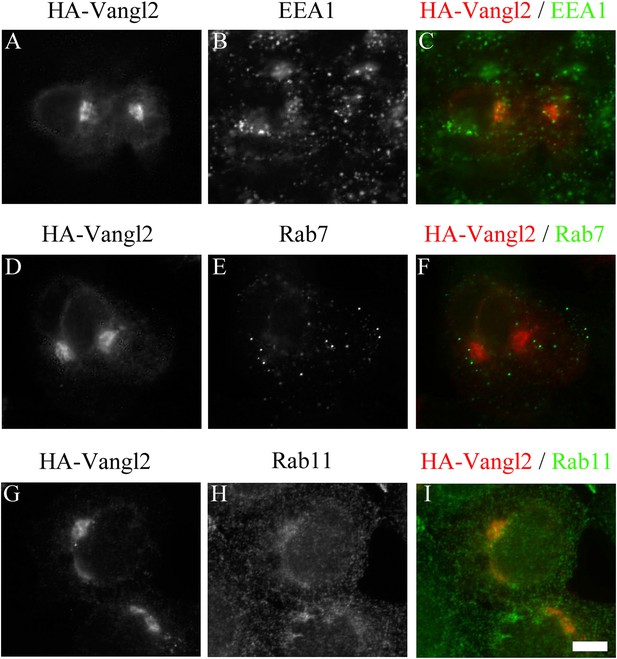
Juxtanuclear accumulated Vangl2 in Arfrp1 knockdown cells is not colocalized with endosomal markers.
HeLa cells were transfected with siRNA against Arfrp1 and re-transfected after 48 hr with a plasmid encoding HA-Vangl2. After an additional 24 hr, cells were immunofluorescently labeled to evaluate coincident localization with HA-Vangl2 and EEA1 (A)–(C), HA-Vangl2 and Rab7 (D)–(F) and HA-Vangl2 and Rab11 (G)–(I). Size bar = 10 μm.
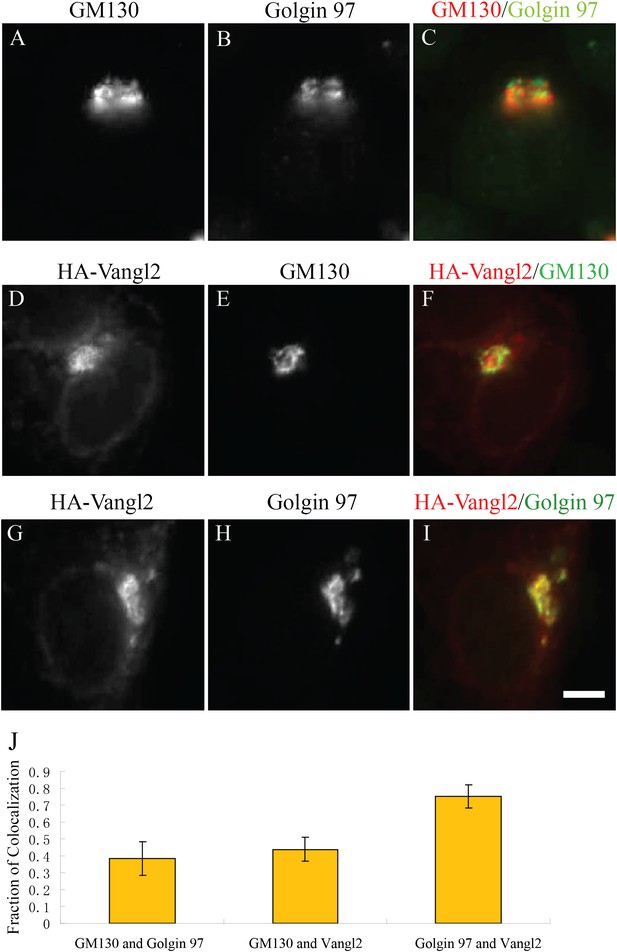
Juxtanuclear accumulated Vangl2 in Arfrp1 knockdown cells colocalizes with Golgin 97 more than with GM130.
(A)–(I) HeLa cells were transfected with siRNA against Arfrp1 and re-transfected after 48 hr with plasmid encoding HA-Vangl2. After an additional 24 hr, cells were immunofluorescently labeled to evaluate coincident localization with Golgin 97 and GM130 (A–C), HA-Vangl2 and GM130 (D–F) and HA-Vangl2 and Golgin 97 (G–I). Size bar = 10 μm. (J) Colocalization was quantified by analyzing the average value of the fraction of each marker's area that coincided with the other marker (mean ± SD; >15 cells each).
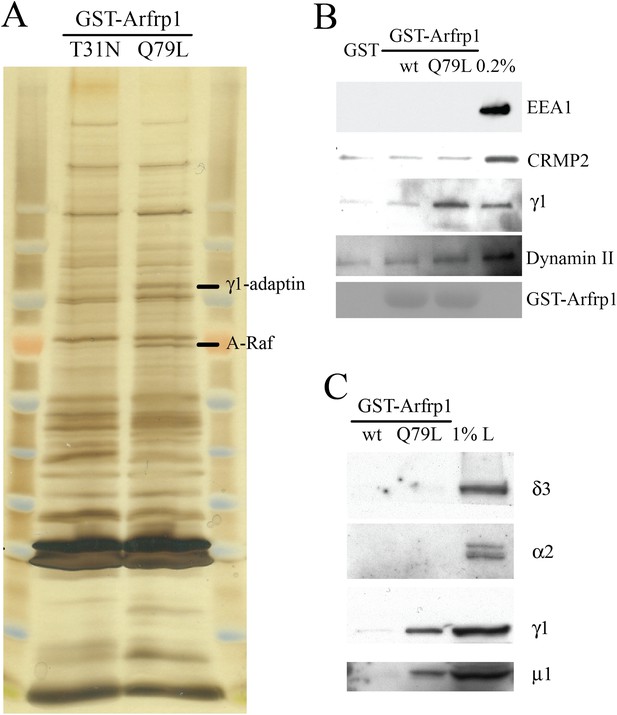
Subunits of AP-1 preferentially interact with the GTP-bound Arfrp1.
(A) Bovine brain cytosol was incubated with purified GDP-loaded dominant negative form (T31N) or GTPγS-loaded dominant active form (Q79L) of GST-Arfrp1. After incubation, the eluted fraction was resolved by SDS-PAGE and silver stained. Protein identification in the indicated gel slice performed by mass spectrometry revealed γ1-adaptin and serine/threonine-protein kinase (A-Raf) respectively. (B),(C). Bovine brain cytosol was incubated with purified GDP-loaded GST-Arfrp1 (wt) or GTPγS-loaded GST-Arfrp1 (Q79L). After incubation, the entire sample of bound γ1-adaptin, μ1-adaptin and other indicated proteins was analyzed by immunoblot.
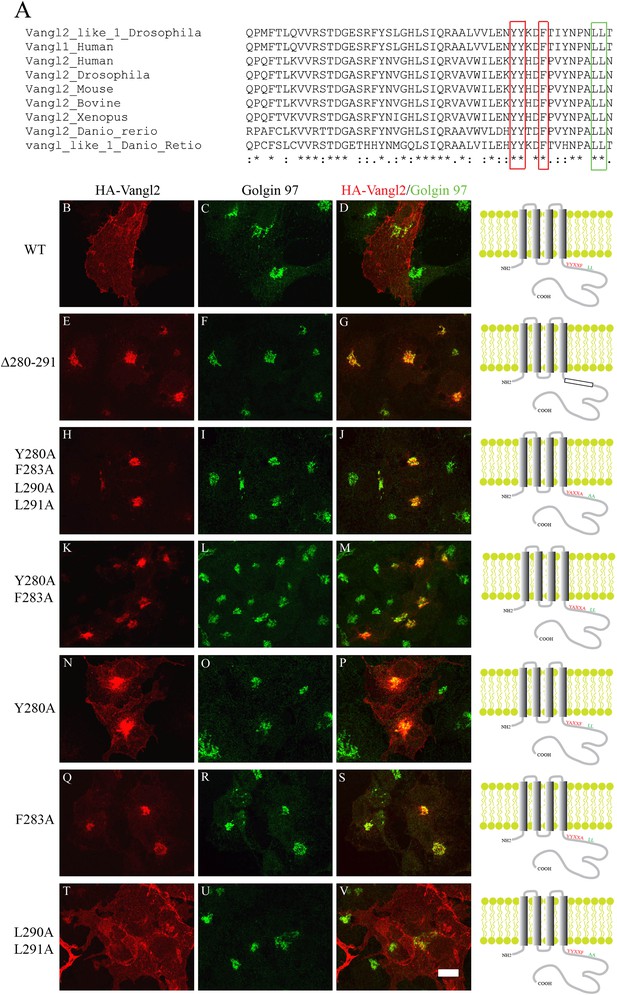
TGN export of Vangl2 depends on the conserved YYXXF sorting motif in the C-terminal cytosolic domain.
(A) Sequence alignment of Vangl1 and Vangl2 from different species indicates that Vangl2 C-terminal cytosolic domain contains a conserved YYXXF sorting motif. (B)–(V) COS7 cells were transiently transfected with plasmids encoding HA-Vangl2 wild type (B–D) or the indicated mutant constructs (E–V). At day 1 after transfection, the cells were analyzed by indirect immunofluorescence using antibodies against HA tag and Golgin 97. Note the contrast in panel N was adjusted to reveal the weak surface pattern of Vangl2. Size Bar = 10 μM.
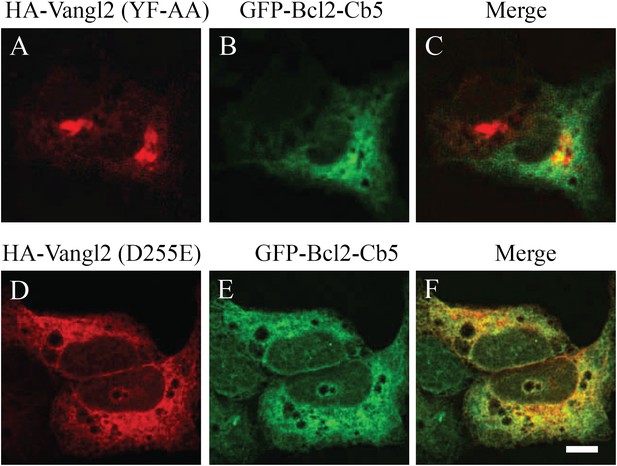
Vangl2 tyrosine mutants are not colocalized with the ER marker.
COS7 cells were co-transfected with plasmids encoding the ER marker (GFP-Bcl2-Cb5) and the indicated HA-Vangl2 mutant construct. At day 1 after transfection, colocalization between GFP-Bcl2-Cb5 and the indicated Vangl2 mutant construct was analyzed by immunofluorescence. Size bar = 10 μm.
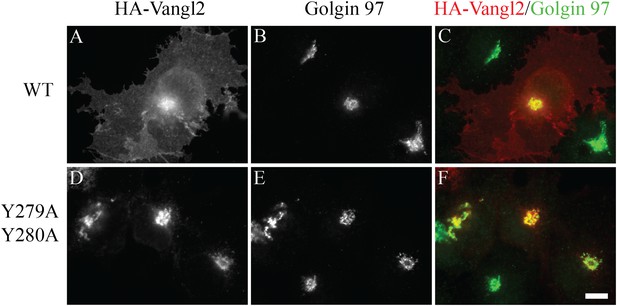
Vangl2 Y279A Y280A is blocked at the TGN.
COS7 cells were transfected with HA-Vangl2 wild type (A)–(C) or HA-Vangl2 (Y279A, Y280A) (D)–(F). After transfection for 24 hr, cells were analyzed by immunofluorescence using anti-HA and anti-Golgin 97 antibody. Size bar = 10 μm.
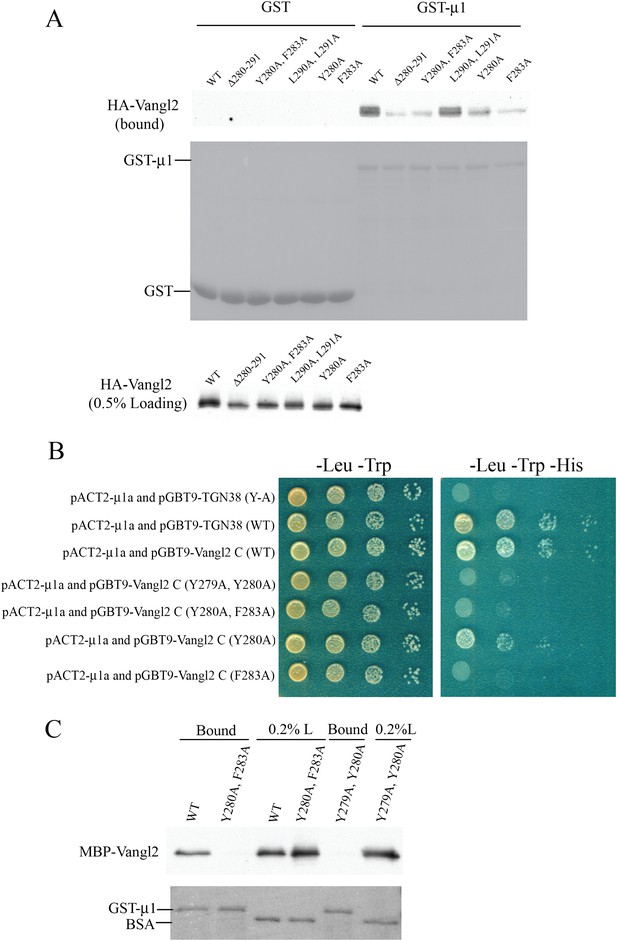
μ1-adaptin directly interacts with Vangl2 C-terminal cytosolic domain in an YYXXF-motif dependent manner.
(A) Cell lysates from COS7 cells transiently transfected with plasmids encoding HA-Vangl2 wild-type or the indicated Vangl2 mutant constructs were incubated with glutathione beads bearing similar amounts of GST or GST-μ1. The entire sample of bound HA-Vangl2 was evaluated by immunoblotting with anti-HA antibody. (B) Yeast two-hybrid analyses recapitulated the results of the GST-pull down assay. Serial dilutions of the yeast colonies co-expressing the indicated constructs were dotted on the correspondent selective media. Pictures were taken after 3 days of growth. (C) Purified MBP-Vangl2 C-terminus wild type, or the indicated mutant constructs were incubated with glutathione beads bearing GST-μ1. The entire sample of bound MBP-Vangl2 was evaluated by immunoblot.
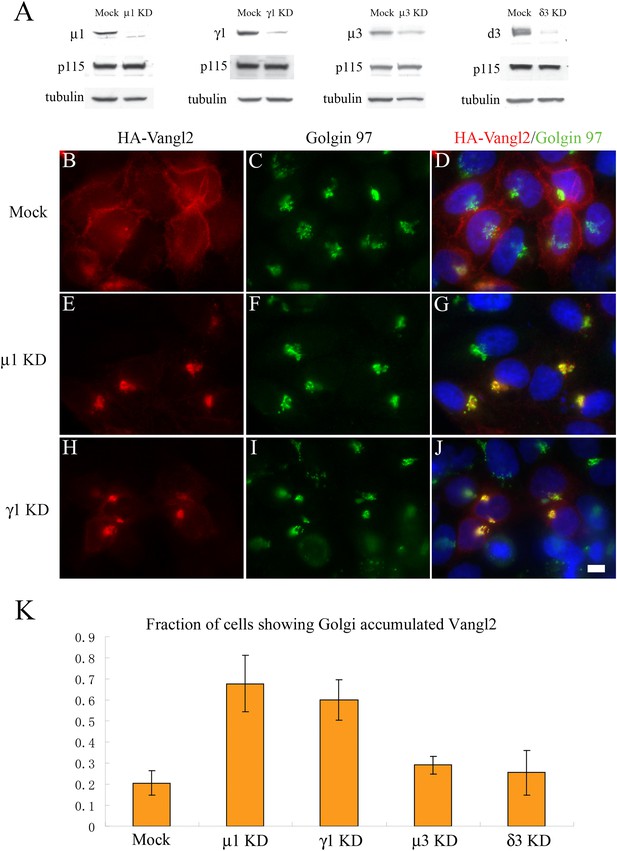
Knockdown of μ1-adaptin or γ1-adaptin accumulates Vangl2 at the TGN.
(A) HeLa cells were mock transfected or transfected with siRNA against the indicated subunit of the AP-1 or AP-3 complex. At day 3 after transfection, total cell lysates were analyzed by immunoblotting with antibody against the indicated adaptin subunits or, as loading controls, p115 and tubulin. (B)–(J) HeLa cells were mock transfected (B–D) or transfected with siRNAs against μ1-adaptin (E–G) or γ1-adaptin (H–J) and re-transfected after 48 hr with plasmid encoding HA-Vangl2. After an additional 24 hr, cells were analyzed by immunofluorescence. Size bar = 10 μM. (K) Quantification of the fraction of cells showing Golgi-accumulated Vangl2 (N = 3; >150 cells expressing lower levels of Vangl2 counted for each experiment).
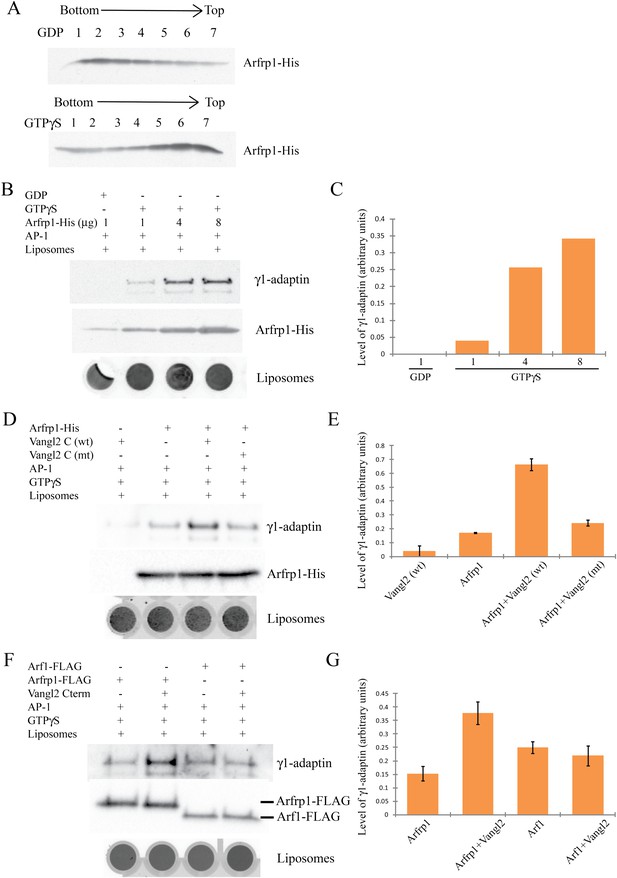
Arfrp1 directly recruits purified AP-1 complex to liposomes and this process is stimulated by Vangl2 C-terminal cytosolic domain.
(A) Purified Arfrp1-His was incubated with liposomes labeled with Texas Red-PE in the presence of GDP or GTPγS. After centrifugation, fractions were collected from the bottom to the top and analyzed by immunoblotting using anti-His antibody. (B),(C). Liposomes were sequentially incubated with Arfrp1-His at the indicated concentration in the presence of GDP or GTPγS, then with purified AP-1 complex. After centrifugation, the top fractions were collected, scanned to reveal fluorescence in the Texas Red channel as an indicator of the amount of liposomes and analyzed by immunoblotting using anti-His and anti-γ1 antibodies (B) and the levels of γ1-adaptin normalized to the amount of lipids were quantified (C). (D),(E). Liposomes were sequentially incubated with Arfrp1-His alone or Vangl2 cytosolic domain alone or both, then with purified AP-1 complex. After centrifugation, the top fractions were collected, scanned to reveal fluorescence in the Texas Red channel, and analyzed by immunoblotting using anti-γ1 and anti-His antibodies (D) and the levels of γ1-adaptin normalized to the amount of lipids were quantified (E, N =2). (F),(G). Liposomes were sequentially incubated with Arfrp1-FLAG or Arf1-FLAG in the presence or absence of Vangl2 cytosolic domain, then with purified AP-1 complex. After centrifugation, the top fractions were collected, scanned to reveal fluorescence in the Texas Red channel and analyzed by immunoblotting using anti-γ1 and anti-FLAG antibodies (F) and the levels of γ1-adaptin normalized to the amount of lipids were quantified (G, N = 3).
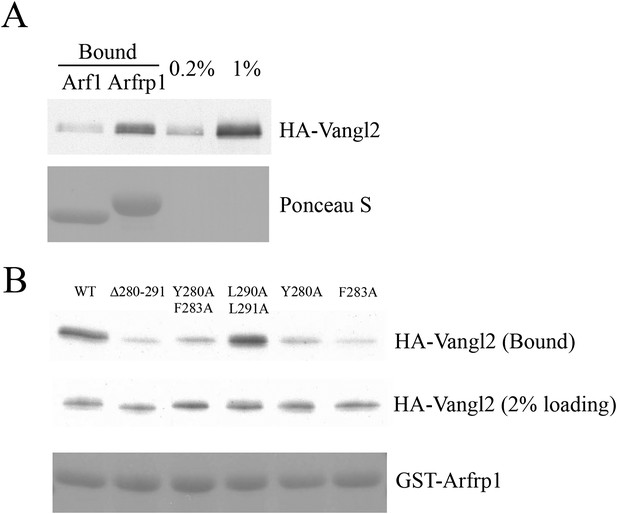
Sorting signal-dependent binding of Arfrp1 to Vangl2 in cell lysates; Vangl2 binds Arfrp1 more efficiently than Arf1.
(A) Cell lysates from COS7 cells transiently transfected with plasmids encoding HA-Vangl2 were incubated with glutathione beads bearing similar amounts of GTPγS-loaded GST-Arf1 or GST-Arfrp1. After incubation, the entire sample of bound HA-Vangl2 was detected by immunoblot. (B) Cell lysates from COS7 cells transiently transfected with plasmids encoding Vangl2 wild type or the indicated Vangl2 mutant constructs were incubated with glutathione beads bearing similar amount of GTPγS-loaded Arfrp1. The entire sample of bound HA-Vangl2 was evaluated by immunoblot.
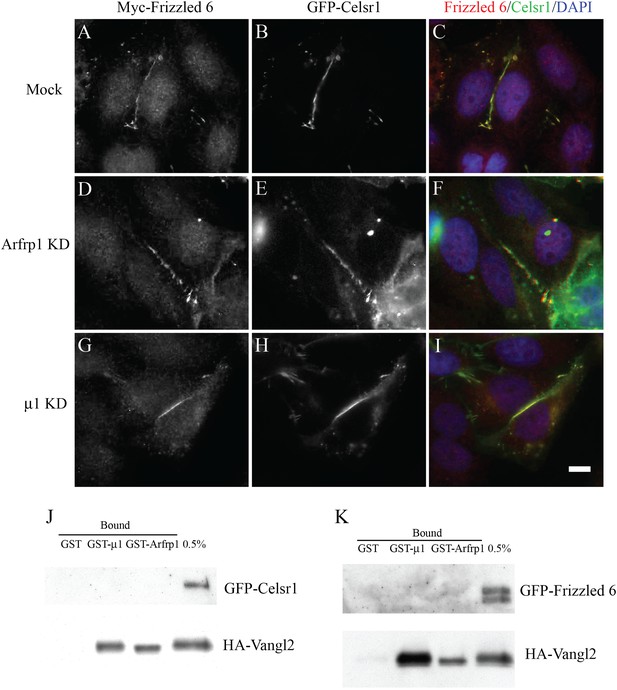
TGN export of Frizzled-6 and Celsr1 is independent of the Arfrp1/AP-1 machinery.
(A)–(I). HeLa cells were either mock transfected (A–C) or transfected with siRNA against Arfrp1 (D–F) or μ1-adaptin (G–I) and re-transfected after 48 hr with plasmids encoding GFP-Celsr1 and Myc-Frizzled 6. After an additional 24 hr, cells were analyzed by immunofluorescence. Size bar = 10 μm. (J),(K). Cell lysates (250 μl) containing 1 mg/ml proteins from COS7 cells co-transfected with HA-Vangl2 and GFP-Celsr1 (J) or HA-Vangl2 and GFP-Frizzled 6 (K) were incubated with glutathione beads bearing 1 μg of GST, GTPγS-loaded GST-Arfrp1 or GST-μ1. The entire sample of bound HA-Vangl2, GFP-Celsr1 or GFP-Frizzled 6 were detected by immunoblot.
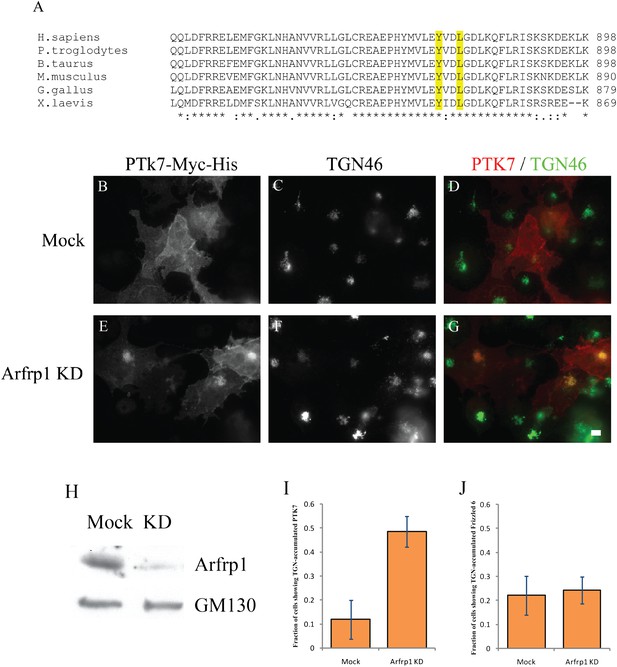
Arfrp1 regulates TGN export of PTK7.
(A) Sequence alignment of PTK7 from different species reveals a conserved tyrosine sorting motif in its predicted C-terminal cytosolic domain. (B)–(G) COS7 cells were transfected with control siRNA or siRNA against Arfrp1 and re-transfected after 48 hr with plasmids encoding PTK7-Myc-His. After an additional 24 hr, cells were incubated at 20°C in the presence of 30 μg/ml cyclohexmide for 4 hr then shifted to 32°C for 90 min. After incubation, cells were analyzed by immunofluorescence using antibodies against His and TGN46. Size bar = 10 μm. (H) COS7 cell lysates from cells transfected with control siRNA or siRNA against Arfrp1 were analyzed by immunoblotting with anti-Arfrp1 antibody and, as a loading control, anti-GM130 antibody. (I) The fraction of cells showing TGN-accumulated PTK7 was quantified after incubation at 32°C (mean ± SD; N = 3; over 150 cells were counted for each group). (J) Similar siRNA knockdown and temperature shift experiments were performed in COS7 cells transfected with HA-Frizzled 6. The appearance of TGN-accumulated HA-Frizzled 6 was quantified in cells treated with control siRNA or siRNA against Arfrp1 after an incubation at 32°C (mean ± SD; N = 2; over 100 cells were counted for each group).
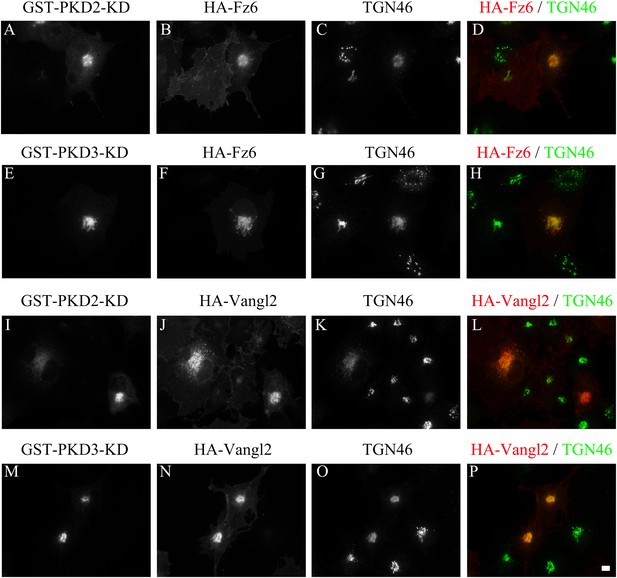
TGN export of Vangl2 and Frizzled 6 is protein kinase D dependent.
COS7 cells were co-transfected with GST-PKD2-KD and HA-Frizzled 6 (A)–(D), GST-PKD3-KD and HA-Frizzled 6 (E)–(H), GST-PKD2-KD and HA-Vangl2 (I)–(L) or GST-PKD3-KD and HA-Vangl2 (M)–(P). Day 1 after transfection, cells were analyzed by immunofluorescence using anti-HA, anti-TGN46 and anti-GST antibodies. Size bar = 10 μm.
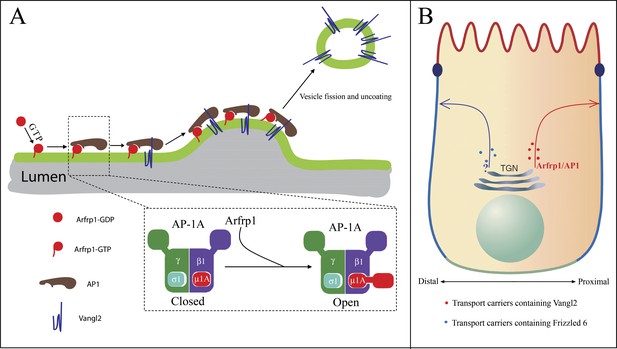
Proposed model.
(A) Model depicting Arfrp1- and AP-1-mediated TGN sorting of Vangl2. Arfrp1 is recruited to TGN membranes upon GTP binding, possibly mediated by a TGN localized GEF. Subsequently, GTP-bound Arfrp1 recruits AP-1 to TGN membranes. GTP-bound Arfrp1 also promotes an open conformation of AP-1 that directly interacts with the tyrosine motif on Vangl2 cytosolic domain, thereby enriching Vangl2 in budding vesicles. Binding of Vangl2 cytosolic domain to AP-1, in turn, stabilizes the membrane association of AP-1 to allow sufficient time for AP-1 polymerization (possibly with clathrin as a coat outer layer) and vesicle budding. This model is consistent with reports showing that tyrosine sorting motifs promote membrane recruitment of AP-1 mediated by Arf1 (Crottet et al., 2002; Lee et al., 2008). (B) The asymmetrically localized PCP signaling molecules, including Vangl2 and Frizzled 6, are sorted by different sorting machineries for export from the TGN. Differential TGN sorting and polarized trafficking of these signaling receptors may contribute to their asymmetric distribution and the laterally polarized organization of epithelial cells.





