Cancer: Evolution, ovulation and cancer
Cancer of the ovaries is difficult to detect early, hard to treat, and its origins are still controversial. Now, in eLife, Jianjun Sun and Allan Spradling of the Carnegie Institution for Science report new data on an evolutionarily conserved pathway for the regulation of ovarian function in the fruit fly (Drosophila melanogaster), which could advance our understanding of ovarian cancer in humans (Sun and Spradling, 2013).
As with breast cancer, a woman's risk of developing ovarian cancer is increased if she inherits mutant forms of the genes BRCA1 and/or BRCA2. Some women with these mutations choose to have surgery to reduce their cancer risk (Berns and Bowtell, 2012), and studying tissues from these patients has increased our understanding of how the disease progresses. These and other studies have classified ovarian cancers into distinct types, and have also provided cell lines that can be used to study the disease at the molecular and cellular level (Levanon et al., 2010).
Some such studies suggest that cancers with an apparent ovarian origin—particularly high grade serous ovarian cancer—may in fact be cancers of the secretory epithelial cells of the fallopian tube. However, there is evidence that these cells may have an indirect role in some ovarian cancers too; simply tying off, or ligating, the fallopian tubes, which convey eggs from the ovaries to the uterus, decreases the incidence of ovarian cancer (Cibula et al., 2011). Moreover, there is other evidence for a link between ovarian cancer and the regulation of ovulation (Purdie et al., 2003). Although the major hormonal cues for ovulation are well characterized, the role of secretions from epithelial cells lining the fallopian tube in ovulation is poorly understood. However, if these pathways and molecules are conserved between species, the work of Sun and Spradling in Drosophila could allow us to make significant headway in understanding the secretory regulation of ovulation in mammals, and possibly shed new light on the genesis of ovarian cancers.
In both flies and mammals, ovulation usually results in the release of just one egg cell per cycle. In Drosophila, muscle contractions move the egg through the oviduct, where it can be fertilized by sperm stored in the spermathecae, before moving on to the uterus and finally being deposited from the vulva (Figure 1; Middleton et al., 2006). The work of Sun and Spradling builds on their previous research into the development of secretory glands in other parts of the female reproductive system (Allen and Spradling, 2008; Sun and Spradling, 2012). These studies identified the regulatory protein lozenge, which is a member of an evolutionarily conserved family of proteins called Runx, as an important player in secretory system development. And indeed, other members of the Runx family have been implicated in many ovarian cancers (Nevadunsky et al., 2009; Li et al., 2012; Keita et al., 2013).
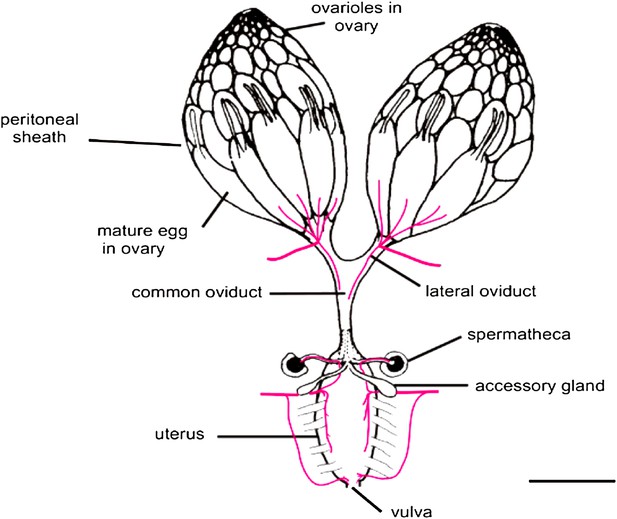
The Drosophila female reproductive tract (figure adapted from Middleton et al., 2006). The mature egg is transported through the oviduct during ovulation and is fertilized by sperm stored in the spermathecae and seminal receptacle (the latter is not shown). The spermathecae and the accessory glands (parovaria) are the major secretory components of the female reproductive system. Sun and Spradling (2013) show that glandular secretions from these structures regulate ovulation and promote sperm storage. The innervation of muscles is shown in magenta. Scale bar: ∼250 μm.
IMAGE: Middleton et al. (2006).
Sun and Spradling's experiments now tease apart the multiple roles of the secretory glands to home in on those aspects relevant to sperm storage and ovulation. They do this in two ways. First, they alter the number of secretory cells in the reproductive tract and show that there are adverse consequences for sperm storage and sperm health, as well as for ovulation. In fruit flies, sperm are stored inside the spermathecae and the seminal receptacle within a few hours of mating. Sun and Spradling find that reducing the number of secretory cells (to fewer than 25) prevents the accumulation of sperm in the spermathecae but not in the seminal receptacle, suggesting that distinct mechanisms target and/or retain sperm in each location. However, even sperm that do accumulate in the receptacle aggregate abnormally, showing that secretions are also essential for the health of the stored sperm.
When Sun and Spradling modulate well-known ‘canonical' molecular pathways controlling secretion in these cells, sperm storage in the spermathecae is reduced. However, abolishing these canonical secretory pathways does not affect ovulation, even though a reduction in the number of secretory cells does; this suggests that the effect of altering secretory cell number on ovulation is likely to be mediated by one or more secretions through non-canonical pathways.
These findings raise important questions about our understanding of ovarian function and cancer. Does the number and/or secretory ability of fallopian tube epithelial cells correlate with either ovulatory capacity or the ability of tubal epithelium to bind and retain sperm, and could this explain some types of infertility? Might the secretory ability of cells within the female reproductive system be linked to their reported high propensity to DNA damage, and so directly mediate their contribution to ovarian cancer? Also, if the number of secretory cells and/or the integrity of non-canonical secretory pathways are regulators of ovulation, abnormalities in these may contribute to abnormal ovulation, which could, in turn, influence the likelihood of cancer.
Consistent with an indirect role for secretory cells in some ovarian cancers, it is noteworthy that members of the Runx family of gene regulatory proteins, familiar figures in ovarian cancers, are reported to regulate secretion (Little et al., 2012). Such a possibility would connect elegantly with the reduced frequency of ovarian cancers upon fallopian tube ligation. But which genes and proteins control these secretory pathways? There are now enough genetic and molecular tools available—in both fruit fly and mammalian systems—to make identifying these components a tractable endeavor. Isolating secretions from mammalian fallopian tubes and examining their effects on Drosophila ovulation could be a fruitful exercise, as could genetic approaches that screen directly for ovulation defects. The work of Sun and Spradling thus opens up new avenues for the genetic and molecular analysis of the properties and functions of secretory cells in the reproductive tract, and their potential role in evolutionarily conserved aspects of carcinogenesis.
References
-
Underlying mechanisms of ovarian cancer risk reduction after tubal ligationActa Obstet Gynecol Scand 90:559–563.https://doi.org/10.1111/j.1600-0412.2011.01114.x
-
Ovulation and risk of epithelial ovarian cancerInt J Cancer 104:228–232.https://doi.org/10.1002/ijc.10927
Article and author information
Author details
Publication history
- Version of Record published: April 16, 2013 (version 1)
Copyright
© 2013, VijayRaghavan and Rath
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 955
- views
-
- 67
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Cell Biology
Collective cell migration is fundamental for the development of organisms and in the adult for tissue regeneration and in pathological conditions such as cancer. Migration as a coherent group requires the maintenance of cell–cell interactions, while contact inhibition of locomotion (CIL), a local repulsive force, can propel the group forward. Here we show that the cell–cell interaction molecule, N-cadherin, regulates both adhesion and repulsion processes during Schwann cell (SC) collective migration, which is required for peripheral nerve regeneration. However, distinct from its role in cell–cell adhesion, the repulsion process is independent of N-cadherin trans-homodimerisation and the associated adherens junction complex. Rather, the extracellular domain of N-cadherin is required to present the repulsive Slit2/Slit3 signal at the cell surface. Inhibiting Slit2/Slit3 signalling inhibits CIL and subsequently collective SC migration, resulting in adherent, nonmigratory cell clusters. Moreover, analysis of ex vivo explants from mice following sciatic nerve injury showed that inhibition of Slit2 decreased SC collective migration and increased clustering of SCs within the nerve bridge. These findings provide insight into how opposing signals can mediate collective cell migration and how CIL pathways are promising targets for inhibiting pathological cell migration.
-
- Cell Biology
- Neuroscience
Alternative RNA splicing is an essential and dynamic process in neuronal differentiation and synapse maturation, and dysregulation of this process has been associated with neurodegenerative diseases. Recent studies have revealed the importance of RNA-binding proteins in the regulation of neuronal splicing programs. However, the molecular mechanisms involved in the control of these splicing regulators are still unclear. Here, we show that KIS, a kinase upregulated in the developmental brain, imposes a genome-wide alteration in exon usage during neuronal differentiation in mice. KIS contains a protein-recognition domain common to spliceosomal components and phosphorylates PTBP2, counteracting the role of this splicing factor in exon exclusion. At the molecular level, phosphorylation of unstructured domains within PTBP2 causes its dissociation from two co-regulators, Matrin3 and hnRNPM, and hinders the RNA-binding capability of the complex. Furthermore, KIS and PTBP2 display strong and opposing functional interactions in synaptic spine emergence and maturation. Taken together, our data uncover a post-translational control of splicing regulators that link transcriptional and alternative exon usage programs in neuronal development.





