Diversity and plasticity in Rab GTPase nucleotide release mechanism has consequences for Rab activation and inactivation
Figures
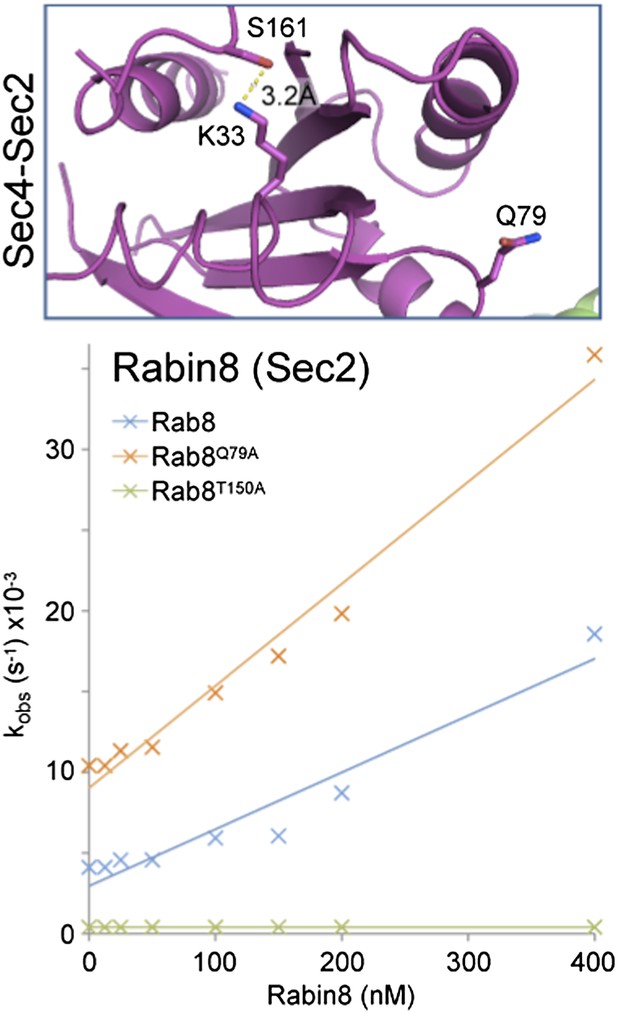
Role of switch II residues in Rab GEF complexes.
(A) The crystal structure of Ras with its exchange factor SOS highlighting the interaction of the Ras P-loop lysine 16 with the Ras switch II glutamate 62. Dotted yellow lines indicate potential Ras P-loop lysine interactions. (B) Portions of Rab structures from Rab–RabGEF complex crystal structures are shown for Rab35-DENND1 and (C) Rab1-DrrA. (D) Ypt1 (budding yeast Rab1) TRAPP and (E) Rab21-Vps9/Rabex complexes are shown. The Rab is indicated in grey while the GEF is depicted in green. Switch II residues are coloured according to their position for ease of reference.
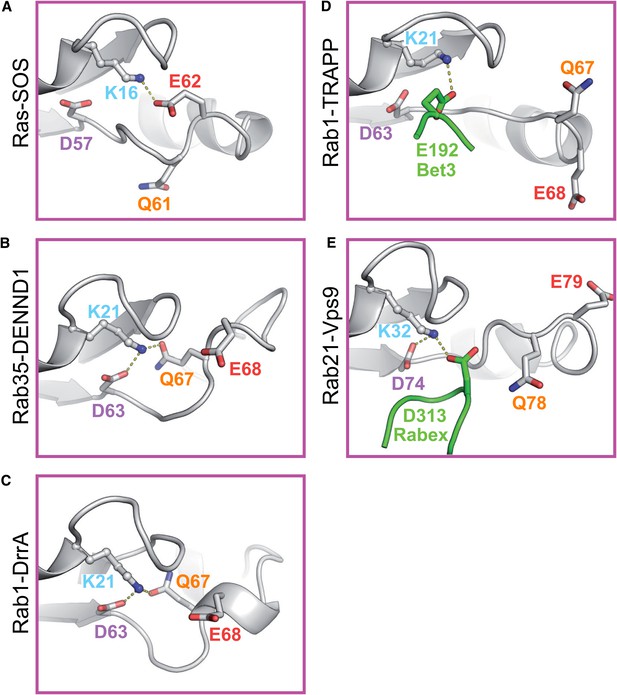
Distinct roles for switch II residues in GEF-mediated nucleotide exchange independent of the basal nucleotide release pathway.
(A) Initial rates of nucleotide exchange as a function of GEF concentration are plotted for Rab35-DENND1, (B) Rab1-DrrA, (C) Rab5-Rabex and (D) Rab1-TRAPP. Wild type and mutant Rabs were used as indicated; curves are colour coded as in Figure 1 according to the position in the switch II region predicted to be important for GEF-mediated nucleotide release. Wild type full-length GEFs were used for DENND1, DrrA, Rabex and TRAPP, as well as the Rabex D313A mutant, and the TRAPP Bet3 E192A/D193A mutant.
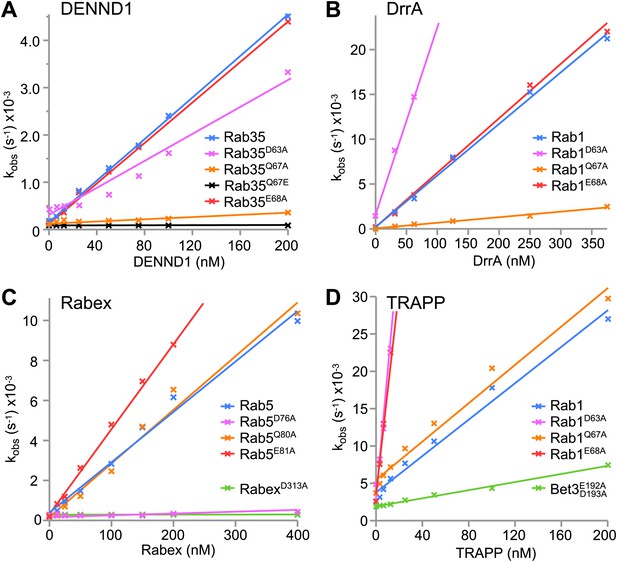
Differential requirements for the Rab switch II glutamine in GAP-stimulated GTP hydrolysis.
(A) Rab GTP hydrolysis as a function of GAP concentration are plotted for Rab35-TBC1D10, (B), and Rab1-TBC1D20 (C) Rab5-RUTBC3. Both wild type and switch II glutamine mutant Rabs were used. Basal GTP hydrolysis of purified Rabs in the absence of the cognate GAP is shown in the bar graph insets (mean +/− the deviation from the mean, n = 2).
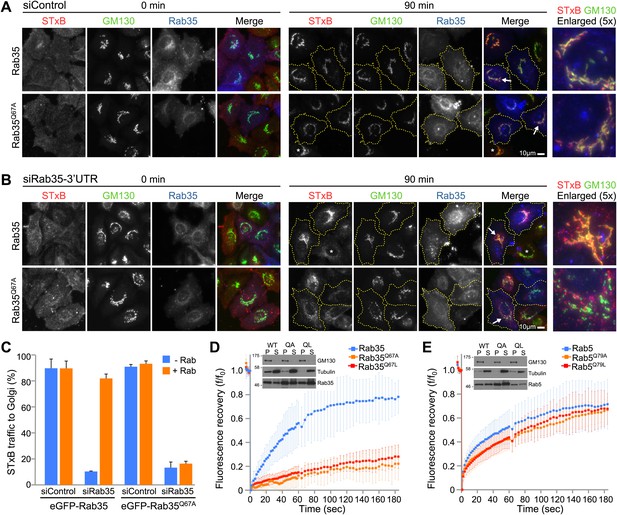
The glutamine switch II mutant Rab35 fails to support Shiga toxin transport from the cell surface to the Golgi.
(A) Shiga toxin B (STxB) uptake assays were performed for 0 and 90 min in HeLa cells expressing either Rab35, or (B) the Rab35Q67A mutant. Endogenous Rab35 was depleted using siRNA directed to the 3′-UTR or a mock depletion performed using a non-targeting control duplex. Cells were stained with a GM130 antibody to mark the Golgi. Scale bar is 10 µm. Cells outlined in yellow dotted lines in the 90 min timepoint express GFP-Rab35 or Rab35Q67A, and asterisks mark non-transfected cells. Arrowheads mark those cells shown in the enlarged panels to the right. (C) Delivery of Shiga toxin into the Golgi was scored and is plotted in the bar graph (mean +/− deviation from the mean, n = 2). (D) FRAP experiments were performed on cells expressing wild type and Switch II glutamine mutant Rab35 or (E) Rab5 (mean +/− standard deviation from the mean, n = 12). Western blots show the distribution of GFP-Rab35 or GFP-Rab5 in the membrane and cytosol fractions marked by the Golgi membrane protein GM130 and tubulin, respectively.
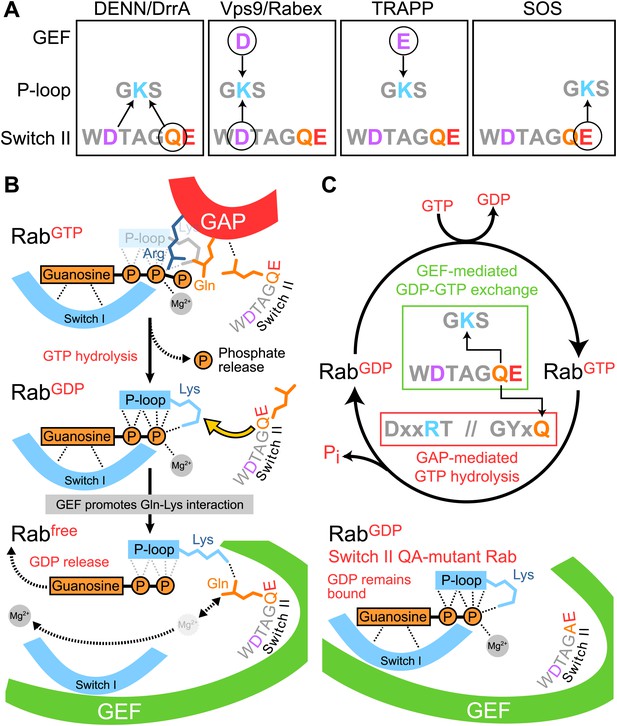
Diversity and plasticity in Rab GTPase nucleotide release mechanism.
(A) A schematic depicting the three different P-loop lysine interactions with the Rab switch II region and GEF, or Ras and the GEF SOS. Circled residues are required for GEF-mediated GDP release. (B) The switch II glutamine is required for both Rab GAP stimulated GTP hydrolysis, and DrrA or DENN GEF mediated nucleotide exchange reactions. GEF interaction with the GTPase results in distortion of switch I and II regions, and reduced affinity for both the guanosine and terminal phosphate of bound GDP. The switch II glutamine interacts with the P-loop lysine to displace the β-phosphate. This does not occur for switch II Q-mutant Rabs and GDP-release therefore fails. Adapted from Figure 5 of Thomas and Wittinghofer 2007 (Thomas et al., 2007). (C) A revised Rab GTPase cycle in which GEF-stimulated activation and GAP-mediated inactivation share a common determinant with respect to switch II.