The Kinesin-12 Kif15 is a processive track-switching tetramer
Figures
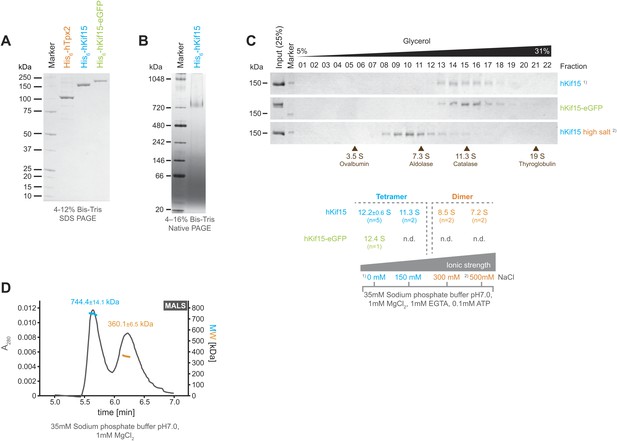
Kif15 is a tetramer.
(A) Coomassie stained SDS-PAGE gel of purified His6-hTpx2, His6-hKif15, and His6-hKif15-GFP. (B) Tetrameric His6-hKif15 on a 4–16% Native-PAGE gel stained with coomassie. Calculated molecular weight: His6-hKif15: 165 kDa. (C) Coomassie stained SDS-PAGE gels of fractions 1–23 out of 25 from 5–40% glycerol gradients loaded with either ∼5 µg His6-hKif15 or His6-hKif15-eGFP at different salt concentration (see table ‘below’ that summarises the apparent S values of hKif15(eGFP) at different salt concentrations). (D) Elution profile (grey line, A280, left y-axis) from a size-exclusion chromatography (SEC) run with subsequent multi angle light scattering (MALS) analysis. Outcome of the MALS analysis for the peaks is presented in coloured lines (blue-hKif15 tetramer, orange hKif15 dimer, MW, right y-axis). Molecular weight ± uncertainty is given above each peak. Please note that the presence of dimeric hKif15 species is specific to the protein preparation used in this experiment only (‘Materials and methods’). Standard preparations do not contain any significant proportion of dimeric hKif15 (see panels B and C this Figure).
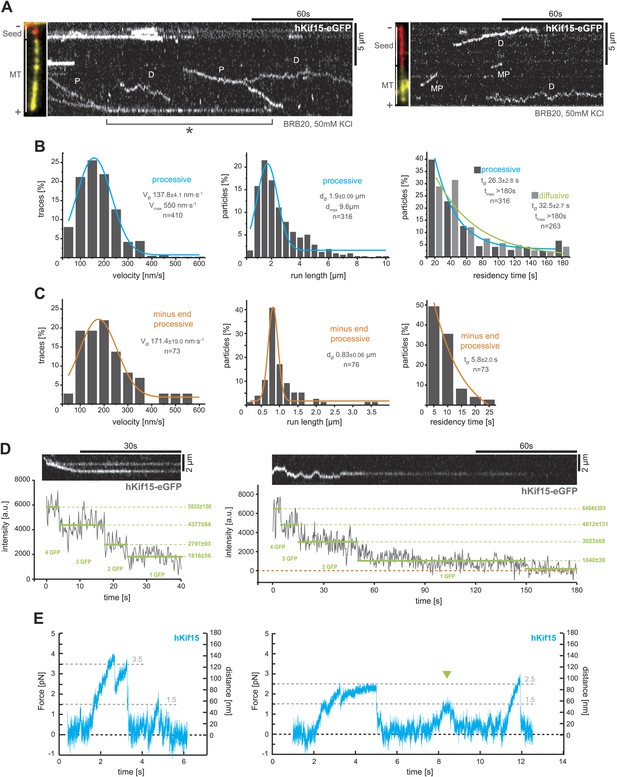
hKif15 is a processive tetramer.
(A) Kymographs showing typical behaviour of eGFP-labelled hKif15 motors on GMP-CPP stabilised microtubules (processive movement–P, diffusion–D, plus-end dwelling–asterix, processive minus-end directed movement–MP). Pictures on the left of each kymograph show orientation of the polarity labelled microtubule. (B) Frequency distributions showing kinetic properties of processively moving plus-end-directed eGFP-labelled hKif15 motors. Coloured lines within the column plots are Gaussian or exponential decay fits. Insets show the respective median ± standard error of mean and maximum value of the distribution as well as its sample size. All values are derived from kymographs as shown in (A). (C) Frequency distributions showing kinetic properties of eGFP-labelled hKif15 motors that move processively to the minus-end of a microtubule. Coloured lines within the column plots are Gaussian or exponential decay fits. Insets show the respective median ± standard error of mean and maximum value of the distribution as well as its sample size. All values are derived from kymographs as shown in (A). (D) Kymographs showing a processively moving hKif15-eGFP motor (left) and a diffusive hKif15-eGFP motor (right) that photo-bleach in three and four steps respectively, indicating presence of four eGFP molecules in each motor. Plot below the kymograph shows the intensity along motor traces in arbitrary units. Intensity had been locally corrected for background intensity. Horizontal green lines within the graphs indicate the fitted average intensity (arbitrary units ± standard deviation) in the respective section of the trace. Photo-bleaching steps were manually defined. Please note that for the last bleaching step in the right kymograph we cannot formally exclude a dissociation event. (E) Example traces showing the movement of a single molecule (hKif15 tetramer) as a function of time (1 ms boxcar filtered). The motor steps out of the trap centre (black dotted line) until it detaches at variable loads between 1.5 and 3.5 pN and reattaches (in the trap centre) for subsequent movements. Movements can also be bidirectional (green arrowhead, compare with Figure 2A).
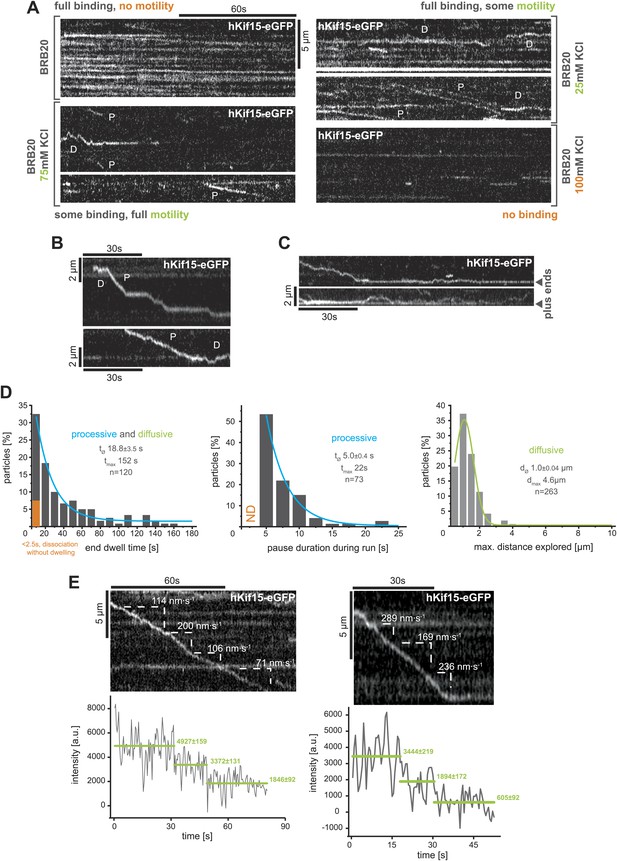
TIRF-based analysis of hKif15 motility.
(A) Kymographs showing the behaviour of hKif15-eGFP motors on GMP-CPP stabilised microtubules in dependency indicated salt concentrations apart from the standard condition used throughout the experiments (BRB20, 50 mM KCl, Figure 2A) (processive movement—P, diffusion—D). (B) Kymographs showing moving hKif15-eGFP motors that convert from diffusive (‘D’) into processive movement (‘P’) or vice versa and show extensive pauses during processive movement (horizontal sections of the trace). (C) Kymographs showing end dwelling hKif15 motors that switch to diffusive movements and roam the end proximal region of the microtubule lattice. (D) Additional frequency distributions showing kinetic properties of processively and diffusively moving eGFP-labelled hKif15 motors. Coloured lines within the column plots are Gaussian or exponential decay fits. Insets show the respective median ± standard error of mean and maximum value of the distribution as well as its sample size. (E) Kymograph showing additional processive moving hKif15-eGFP motors at different velocities that photo-bleach in two steps, indicating the presence of (at least) three eGFP molecules per motor. The speed for each slope-section is given (please compare with velocity distribution in Figure 2B, left panel). Graph below the kymographs show the intensity along motor trace in arbitrary units. Intensity had been locally corrected for background intensity. Horizontal green lines within the graphs indicate the fitted average intensity (arbitrary units ± standard deviation) in the respective section of the trace. Photo-bleaching steps were manually defined. Compare with Figure 2D.
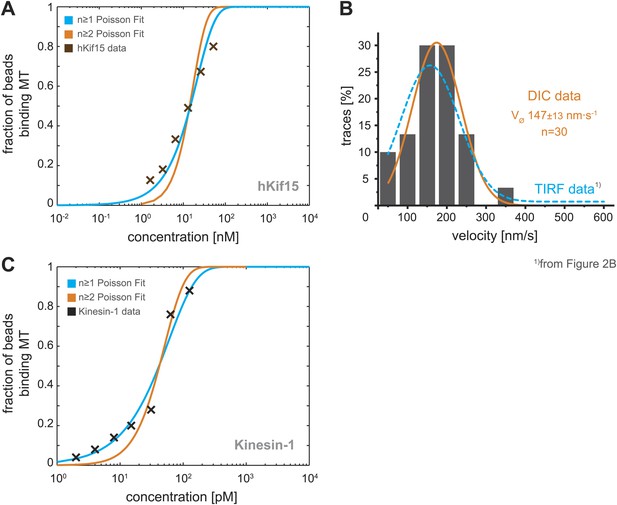
Analysis of bead motility and microtubule-binding in laser trap experiments.
(A) Poisson statistics for the bead-binding probability of n ≥ 1 (blue) and n ≥ 2 (orange) motors fitted to data from a hKif15 dilution series (brown). Number of analysed beads per concentration >50 except for the highest where n = 30 (B) Velocity frequency distributions of hKif15-linked polystyrene beads at zero load followed by DIC microscopy. Orange line within the column plot is a Gaussian fit. Inset shows the respective median ± standard error of mean. Dotted blue line is the TIRF-derived velocity distribution fit from Figure 2B copied for the purpose of comparison. (C) Poisson statistics for the bead-binding probability of n ≥ 1 (blue) and n ≥ 2 (orange) motors fitted to data from a kinesin-1 dilution series (black). Number of analysed beads per concentration n = 25 except for the three lowest where n = 50.
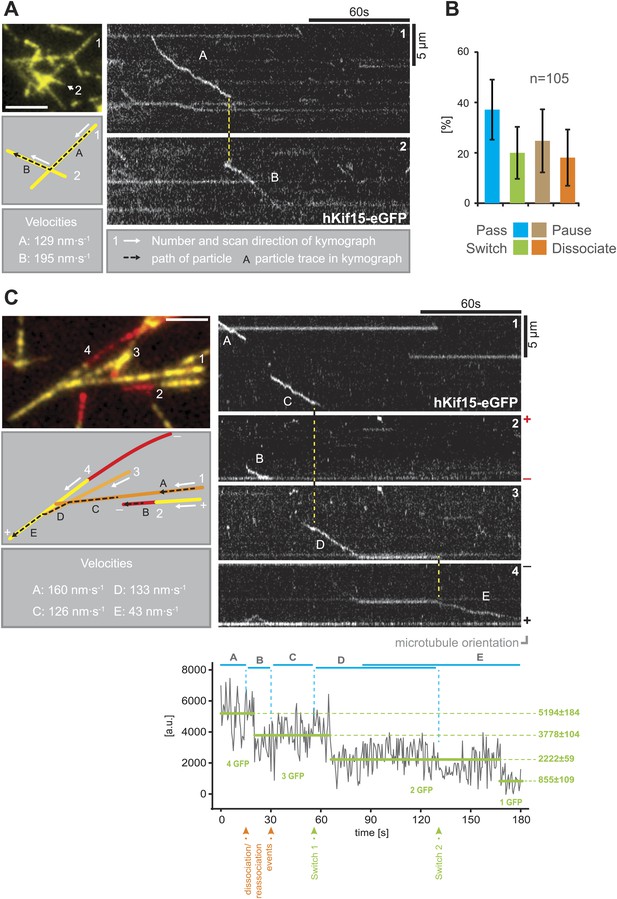
hKif15 effectively switches microtubules at intersections.
(A) Kymographs showing the processive switch of an eGFP-labelled hKif15 motor at a microtubule–microtubule intersection. The image in the upper left gives an overview of the microtubule positons, bar = 5 µm. The schematic below depicts the microtubules whose line scans are depicted as kymographs on the right. The numbering of the microtubules corresponds to that of the respective kymograph and the assigned letters to the traces within the kymograph. White arrows depict the direction of the line-scan; the dotted black arrow depicts the path of the hKif15 motor. Velocities of single trace-sections are given. Please note that the motor never leaves the microtubule lattice during the switch event (yellow dotted line between the kymographs). (B) Quantification of motor behaviour at microtubule intersections. Definitions as following: Pass–motor passes on the same microtubule and continues movement (may include a pause at the intersection); Switch–motor switches the microtubule and continues movement (may include a pause at the intersection); Pause–motor pauses for more than 5 s and does not continue movement after intersection (may dissociate or bleach at some point); Dissociate–motor immediately dissociates at intersection. (C) Essentially as in (A), now following a motor along four microtubules. Movement involves two dissociation/re-association (Kymographs: trace A/trace B, trace B/trace C) and two microtubule switch events (trace C/trace D, trace D/trace E) during switch events the motor never leaves the microtubule lattice (yellow dotted line between the kymographs). Plot below the kymograph shows the intensity along motor traces in arbitrary units. Intensity had been locally corrected for background intensity. Horizontal green lines within the graphs indicate the fitted average intensity (arbitrary units ± standard deviation) in the respective section of the trace. Photo-bleaching steps were manually defined and show that the observed motor bleaches in three steps indicating presence of four eGFP molecules.
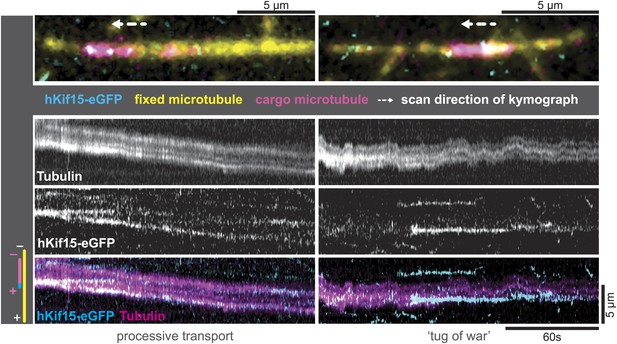
hKif15-eGFP can transport short microtubules as a cargo.
Kymographs showing examples of processive (left) and tug-of-war-type transport (right), with an overview image of the crosslinked microtubules on top. Left: hKif15-eGFP motors drive slow (26 nm•s−1) processive movement of a cargo microtubule. The moving microtubule (magenta) is attached via Kif15 motors (cyan) to a substrate microtubule (yellow) for which the plus-end is orientated left in the overview image on top and at the bottom of the kymograph. hKif15 motors can be seen stably associated with one end of the cargo microtubule, which must be the plus-end because the motors move in a plus-end direction and pause at the end (see Figure 2A, Figure 2—figure supplement 1C, see also schematics to the left). Thus, the cargo microtubule moves plus-end leading towards the plus-end of the substrate microtubule. That is, the microtubules are parallel. Right: hKif15-eGFP motors drive tug-of-war-type movement, characterised by frequent and rapid direction changes during movement.
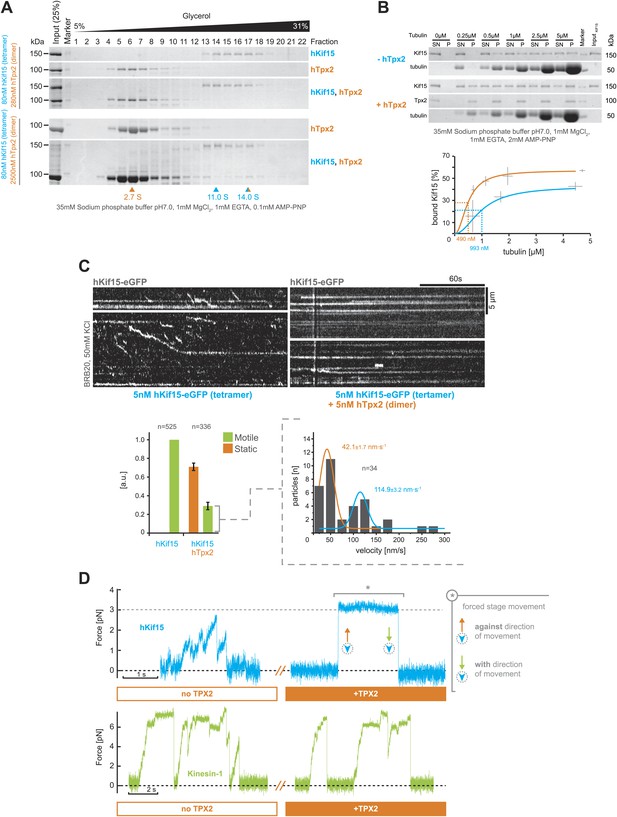
hTpx2 inhibits hKif15 motility by increasing its microtubule affinity.
(A) Formation of a stable hKif15/hTpx2 complex occurs only at low µM concentration of Tpx2. Coomassie stained SDS-PAGE gels showing the first 23 of 25 fractions of a 5–40% glycerol gradient in 35 mM sodium phosphate buffer loaded with the indicated purified proteins alone and in mixture at the indicated concentrations. While hKif15 and hTpx2 do not form a stable complex at nanomolar concentrations, a vast excess of 2.5 µM dimeric hTpx2 drives formation of a hKif15/hTpx2 complex of 14.0S. (B) Above: coomassie stained SDS-PAGE gel of a typical microtubule co-sedimentation experiment of 15 nM tetrameric hKif15 in the presence or absence of 30 nM dimeric hTpx2 at different concentrations of taxol-stabilised microtubules (SN–supernatant, P–pellet). Below: Quantification of pelleted tubulin and bound hKif15 shown above. Crosses indicate the SD of the average from three independent experiments. Deviation in tubulin concentration is due to partial microtubule instability in phosphate buffer at low tubulin concentration and the microtubule stabilising effects of hTpx2 (compare pelleted tubulin at 0.25 µM with and without hTxp2). (C) Kymographs show the effect of 5 nM dimeric hTpx2 on the motility of 5 nM tetrameric hKif15 on GMP-CPP stabilised microtubules. Graph below left shows the fraction of motile and static motors normalised to overall microtubule length and subtracted by the fraction of static motors in the control, which sets motile motors in control to 1. Error bars show SD of three independent experiments. Graph below right shows the velocity distribution of motile motors in the presence of hTpx2, coloured lines are Gaussian fits revealing a bimodal distribution, compare with Figure 2B. (D) Side-by-side comparison of hKif15 (blue) and Drosophila kinesin-1 (green) stepping in the absence and presence of hTpx2 in the laser trap. Stepping traces for each kinesin are from the same bead before flow-in of 36 nM hTpx2 (left trace), after flow-in of hTpx2 (right trace). The asterix in the above right trace marks the deliberate movement of the stage, showing motor maintained attachment to the microtubule.
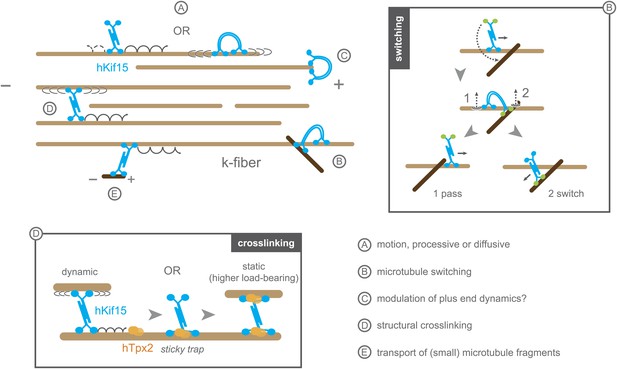
Schematic model summarising the biophysical and biochemical properties of hKif15 and illustrating how the motor may operate within a k-fibre (parallel microtubule bundle).
A–hKif15 can move uni-directionally towards the plus-end (minus-end-directed motion can also occur albeit with lower frequency) by processive stepping or by bi-directionally diffusion along the microtubule lattice. B–hKif15 can switch between microtubule tracks. (box) Model for the sequence of events during switch or pass movements at intersections via a bridge structure of the hKif15 tetramer that resolves into a pass (1) or switch event (2) depending on which motor domain pair detaches first from the microtubule lattice. C–hKif15 has a significant plus-end dwell time and therefore might modulate plus-end dynamics like other k-fibre motors (Stumpff et al., 2012). D–Being a tetramer, hKif15 can crosslink two microtubules and potentially resist sliding of microtubules within the fibre. Note that this link is dynamic as the motor still can step or diffuse within a bundle. (box) Once hKif15 and hTpx2 have formed a complex on the microtubule lattice, this crosslink could become static, thereby forming a fixed structural link in the fibre, which can sustain higher loads than hKif15-only crosslinks. E–hKif15 powers transport of (small) microtubule fragments.
Videos
hKif15 effectively switches microtubules at intersections.
Video of the events summarised in Figure 3A (hKif15-eGFP—cyan, microtubules—yellow). The switching hKif15-eGFP motor is marked by the cyan arrowhead.
hKif15 effectively switches microtubules at intersections.
Video of another hKif15-eGFP motor switching polarity-marked microtubules (hKif15-eGFP—cyan, seed—red, microtubule extension—yellow). The switching motor is marked by the cyan arrowhead. Please note that the motor moves for some time towards the minus-end of the microtubule it switched onto and subsequently changes direction towards the plus-end.
hKif15 effectively switches microtubules at intersections.
Video of the events summarised in Figure 3C (hKif15-eGFP—cyan, seed—red, microtubule extension—yellow). The switching hKif15-eGFP motor is marked by the cyan arrowhead.





