Identification of human TERT elements necessary for telomerase recruitment to telomeres
Figures
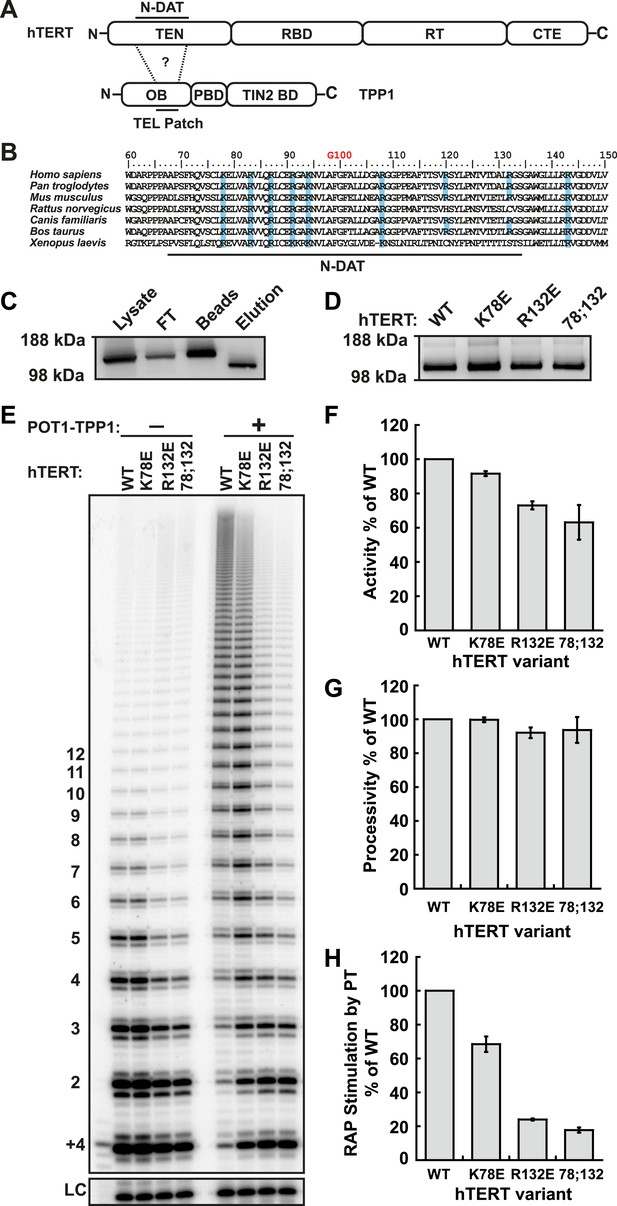
Identification of mutants in the TEN-domain of hTERT that affect the interaction with POT1-TPP1 in vitro but not enzymatic activity.
(A) Domain structures of hTERT and TPP1 proteins. Question mark, proposed interaction between the TEL-patch on TPP1 and the N-DAT region of the TEN-domain of hTERT. In TPP1, the OB-domain (OB) is followed by the POT1-binding domain (PBD), and finally the TIN2-binding domain (TIN2 BD). For hTERT, the Telomerase Essential N-terminal (TEN) domain is adjacent to the RNA binding domain (RBD), which precedes the reverse transcriptase domain (RT) and finally the C-terminal extension (CTE). (B) An alignment of the N-DAT region of the TEN-domain of TERT proteins from selected species. Basic residues conserved in greater than five of the seven species are highlighted blue. The conserved residue G100 is highlighted in orange above the alignment. (C) Western blot, probed for hTERT, showing the immuno-purification of telomerase over-expressed in HEK 293T cells. To monitor relative quantities of hTERT, equal fractions of lysate, flow through (FT), IgG bead capture (capture), and cleaved eluate (elution), were analyzed by SDS-PAGE. (D) Western blot of the relative quantities of wild-type and mutant hTERTs after immuno-purification of the telomerases. (E) Direct telomerase activity assays in the absence and presence of the POT1-TPP1 heterodimer (PT) for wild-type (WT) and mutant telomerases. LC, loading control. +4, oligonucleotide marker corresponding to the addition of the first four nucleotides to primer. Numbers on left, telomeric repeats added. (F–H) Bar graphs representing the quantification of activity, RAP, and RAP stimulation (decay method) by wild-type POT1-TPP1. Values are normalized to WT telomerase, and to WT telomerase with WT POT1-TPP1 for RAP stimulation (n = 3, Mean ± SD).

Additional TEN-domain mutants tested in this study.
(A) Direct telomerase assays of additional hTERT TEN-domain mutants (all with WT hTR) without (−) and with (+) WT POT1-TPP1. LC and +4 marker as in Figure 1E. Quantification in Table 1. (B) Western blot of the relative quantities of wild-type and mutant hTERTs following immuno-purification.
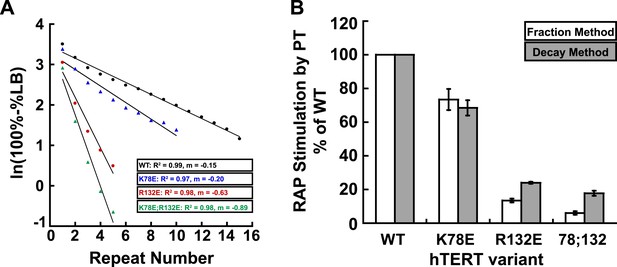
Comparison of methods for quantifying RAP stimulation by POT1-TPP1.
(A) Example of linear regression of data from one replicate that was used to calculate RAP stimulation by POT1-TPP1 in Figure 1H, using the decay method. All four assays included wild-type POT1-TPP1. R2 values and slope of regression are shown. (B) Comparison of RAP stimulation by POT1-TPP1 for data calculated using the decay (shown in Figure 1H) and fraction methods as described in ‘Materials and methods’.
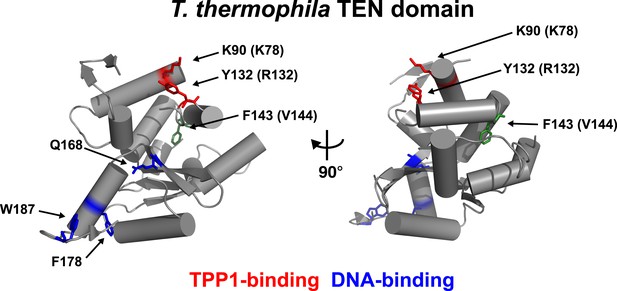
Conserved basic residues K78 and R132 are found in close proximity on the surface of the TEN-domain.
Cartoon representation of the TEN-domain crystal structure from Tetrahymena thermophila (PDB: 2B2A). Amino acid numbers are from Tetrahymena (human counterparts in parentheses). Anchor site (DNA binding) residues are highlighted in blue. The positions of the residues corresponding to human K78 and R132 are highlighted in red, and F143 corresponding to human V144 is highlighted in green.
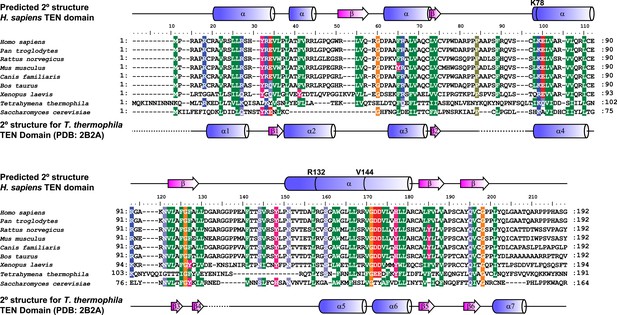
Alignment and secondary structure prediction for human TEN-domain.
Secondary structure prediction for hTERT TEN domain and alignment to selected eukaryotic TERT TEN domains. Human secondary structure prediction made with JPRED3 (Cole et al., 2008), while T. thermophila secondary structure based on crystal structure (PDB: 2B2A). The T. thermophila and S. cerevisiae TEN-domains were aligned to the human TEN-domain using Psi-blast, followed by manual alignment of the remaining species. Similar amino acids are shaded by color.
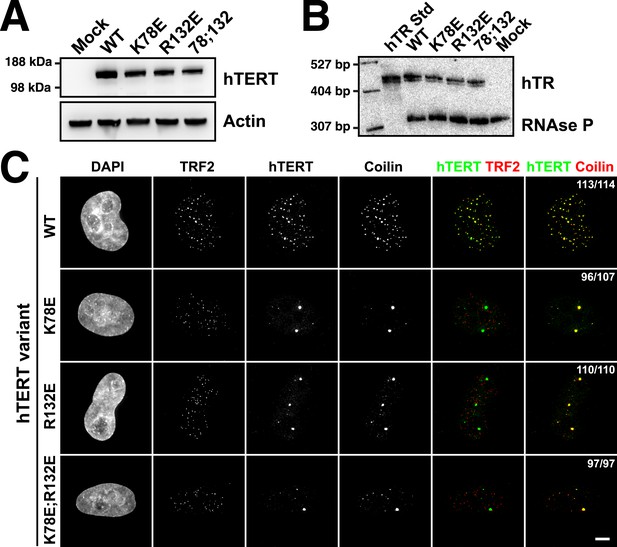
TEN-domain mutations disrupt telomere localization of telomerase.
(A) Western blots of lysates of HeLa cells transfected with expression plasmids for various hTERT alleles and hTR, probed with an antibody against hTERT. Actin was used as a loading control. (B) Northern blots of RNA isolated from HeLa cells transfected with expression plasmids for various hTERT alleles and hTR, using probes for hTR. In vitro transcribed hTR (500 pg) was used as positive control. Blots were probed for RNase P RNA as loading control. (C) Immuno-fluorescence (IF) analysis of HeLa cells transiently transfected with mCherry-hTERT and hTR plasmids. Cells were fixed and probed with antibodies against mCherry, coilin, and TRF2 to visualize telomerase, Cajal bodies and telomeres, respectively. Images were deconvolved. Numbers indicate the fraction of cells analyzed showing the displayed phenotype (scale bar = 5 μm).
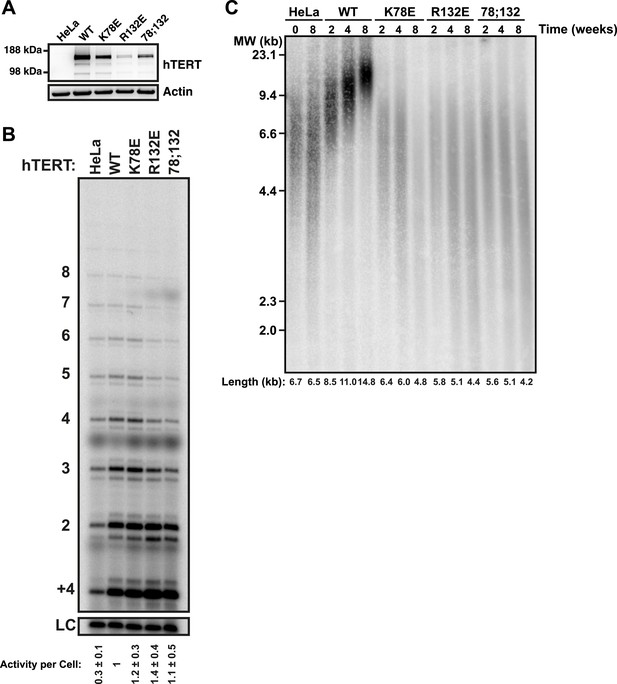
TEN-domain mutants that do not localize to telomeres fail to elongate telomeres in vivo.
(A) Western blot probed for hTERT and for Actin as a loading control, showing hTERT expression in lysates of parental HeLa cells and cell lines stably expressing mCherry-hTERT variants. hTR was not ectopically expressed. (B) Direct enzyme assay of telomerase immuno-purified from lysates, generated using equal number of cells, from parental HeLa or cell lines stably expressing mCherry-hTERT variants. Activity per cell relative to WT hTERT overexpressing cells (n = 4, Mean ± SD, p < 0.05). (C) Telomeric restriction fragment Southern blot of cell lines stably expressing mCherry-hTERT variants over the time course of 8 weeks.
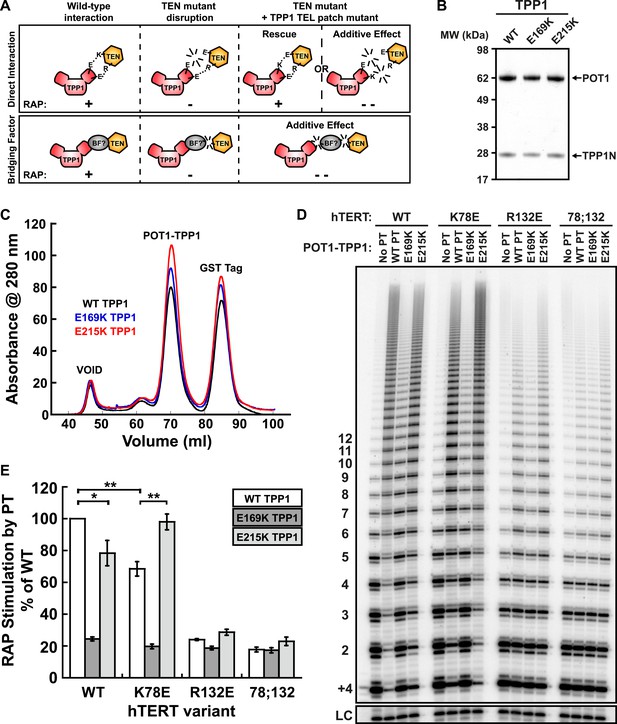
A compensatory mutation in the TEL-patch of TPP1 rescues RAP stimulation of TEN-domain mutant telomerase in vitro.
(A) Schematic of the charge-swap experiment to test the interaction between specific amino acids on the TEL-patch on TPP1 and the N-DAT region of the TEN-domain of hTERT. Predicted experimental outcomes are illustrated for two competing models: a direct TPP1 telomerase interaction and an interaction bridged by a yet-unidentified factor (BF?). (B) Coomassie-stained gel of WT, E169K, and E215K TPP1 co-purified with wild-type POT1. (C) Overlays of the Superdex 200 16/60 sizing column chromatograms for wild-type and mutant TPP1-POT1 complexes. (D) Direct telomerase activity assay to test the rescue of various telomerase containing WT, K78E, R132E, and K78E;R132E mutant hTERTs in the absence of POT1-TPP1 (No PT) or WT, E169K, and E215K TPP1 in complex with wild-type POT1. LC and +4 marker as in Figure 1E. (E) Quantification (decay method) of the RAP stimulation by POT1-TPP1 (PT) relative to the stimulation of wild-type telomerase with wild-type POT1-TPP1 (n = 3, Mean ± SD, *p < 0.05, **p < 0.01, Student's t test).
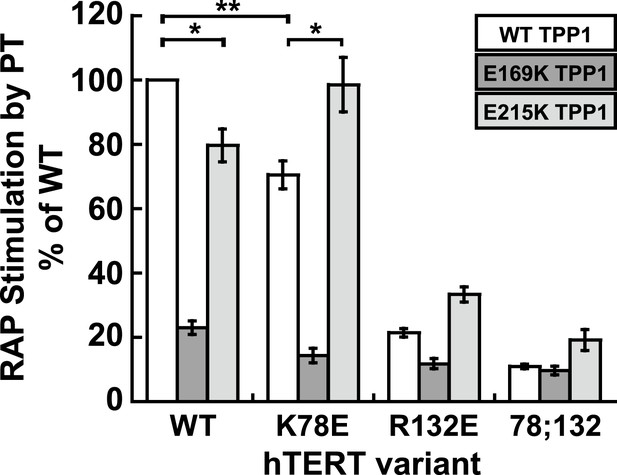
The charge-swap is statistically significant by alternative quantitation methods.
RAP stimulation by PT calculated using the fraction method to quantitate data presented in Figure 5D (n = 3, Mean ± SD, *p < 0.05, **p < 0.01, Student's t test).
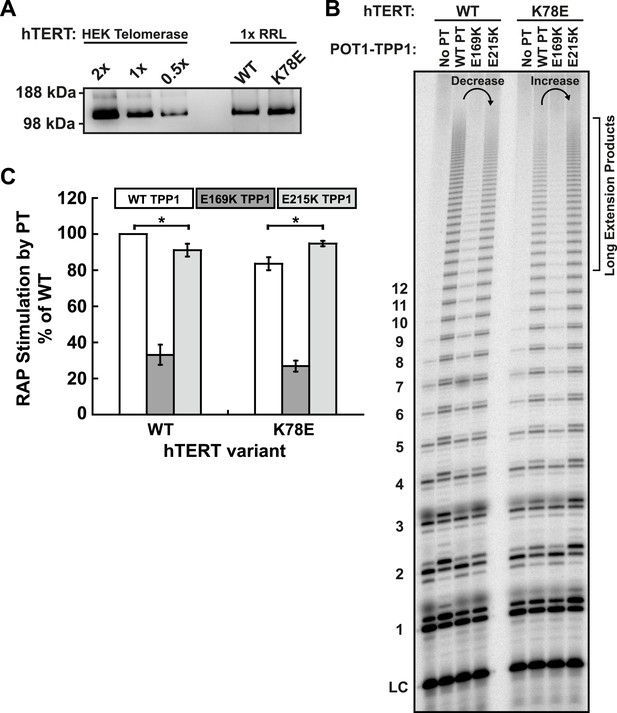
E215K TPP1 rescues RAP stimulation of K78E mutant telomerase produced in RRLs.
(A) Western-blot for hTERT produced in RRLs. Also shown is a serial dilution of overexpressed telomerase immuno-purified from HEK293T cells. 1X signifies amount of telomerase used in direct extension assays. (B) Direct telomerase activity assay to test the rescue of WT and K78E telomerases produced in RRLs, in the absence of POT1-TPP1 (No PT) or WT, E169K, and E215K TPP1 in complex with wild-type POT1. LC as in Figure 1E. Rescue is apparent by looking at the long extension products (>12 repeats), which decrease in intensity from lane 2 to lane 4 for WT hTERT, but then increase from lane 2 to lane 4 for K78E hTERT. (C) Quantification (decay method) of the RAP stimulation by POT1-TPP1 (PT) relative to the stimulation of wild-type telomerase with wild-type POT1-TPP1 (n = 3, Mean ± SD, *p < 0.05).

The IPF allele V144M is deficient in RAP stimulation by TPP1.
(A) Direct telomerase assays of wild type and V144M hTERT telomerases in the presence of WT, E169K, or E215K POT1-TPP1. LC and +4 marker as in Figure 1E. (B) Western blot of the relative quantities of wild-type and V144M hTERTs following immuno-purification. (Note: activity is proportional to hTERT, but RAP is independent of the amount of hTERT in these assays.) (C) Quantification of the RAP stimulation by POT1-TPP1 (PT) relative to the stimulation of wild-type telomerase with wild-type POT1-TPP1 (n = 6, Mean ± SD, **p < 0.01, Student's t test).
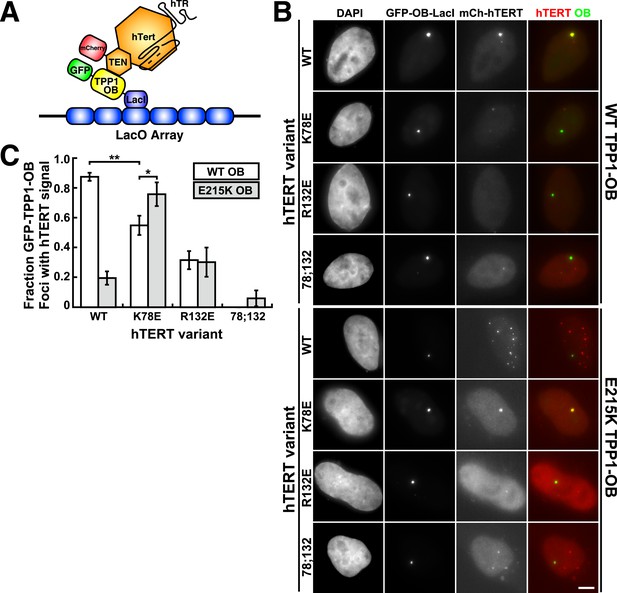
A compensatory mutation in the TEL-patch of TPP1 rescues TEN-domain mutant telomerase binding to the TPP1 OB-domain in vivo.
(A) Model showing the experimental design. Fusion of the OB-domain of TPP1 to the lac repressor (LacI) recruits the OB-domain to a single non-telomeric chromosomal locus (LacO array), allowing the interaction between telomerase and the TPP1 OB-domain to be assessed by co-localization of GFP (TPP1 OB) and mCherry (hTERT) in cell nuclei. (B) Fluorescence images showing the localization of GFP-TPP1-OB-LacI and mCherry-hTERT fusion proteins in cell nuclei stained by DAPI. Cells were fixed, permeabilized, and stained with DAPI. The intrinsic GFP- and mCherry-fluorescence was used to detect GFP-TPP1-OB-LacI and mCherry-hTERT (scale bar = 5 μm). (C) Quantification of the experiments shown in (B), showing the fraction of nuclei with co-localization of GFP- and mCherry-foci (n = 3, 117–176 nuclei total, Mean ± SD, *p < 0.05, **p < 0.01, Student's t test).

Additional examples of U2OS 2-6-3 cells expressing GFP-TPP1 OB-LacI and mCherry-telomerase.
Fluorescence images showing the localization of GFP-TPP1-OB-LacI and mCherry-hTERT fusion proteins in cell nuclei stained by DAPI. Cells were fixed, permeabilized, and stained with DAPI. The intrinsic GFP- and mCherry-fluorescence was used to detect GFP-TPP1-OB-LacI and mCherry-hTERT (scale bar = 5 μm).
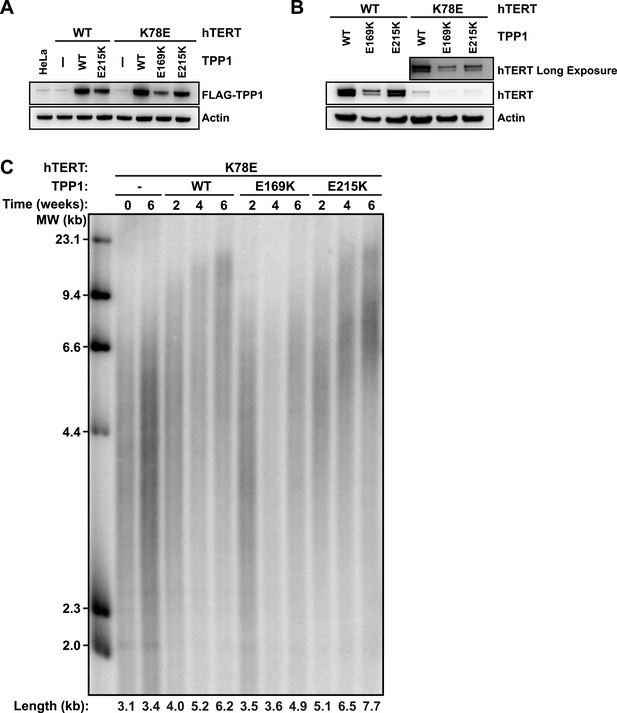
TPP1 E215K rescues telomere maintenance in cells expressing hTERT K78E.
(A) Western blot probed for TPP1-FLAG and for Actin as a loading control, showing TPP1 expression in lysates of parental HeLa cells and cell lines stably expressing TPP1-FLAG and mCherry-hTERT variants. (B) Western blot probed for hTERT and for Actin as a loading control, showing hTERT expression in lysates of cell lines stably expressing TPP1-FLAG and mCherry-hTERT variants. (C) Telomeric restriction fragment Southern blot of cell lines stably expressing K78E mCherry-hTERT and TPP1-FLAG variants over the time course of 6 weeks.
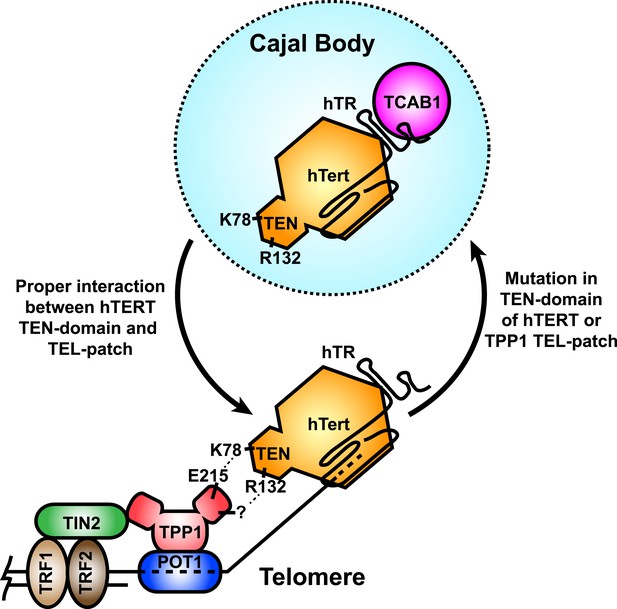
A model for the recruitment of telomerase to telomeres through the direct interaction between the TEL-patch of TPP1 and the hTERT TEN-domain.
Throughout the cell cycle, telomerase associates with TCAB1 and localizes to Cajal bodies. During late S/G2, telomerase is recruited to telomeres through a direct interaction between K78 on the TEN-domain of hTERT and E215 within the TEL-patch of TPP1. R132 in the TEN-domain also participates in the interaction, but the corresponding residue on the TEL-patch and the nature of the interaction remains unknown (?). TPP1 is recruited to telomeres through interaction with TIN2 and further stabilized by the single-stranded DNA-binding protein POT1. TRF1 and TRF2 comprise the double-stranded DNA binding proteins of shelterin. Mutations in the OB-domain of TPP1 or TEN-domain of hTERT that disrupt the interaction are sufficient to prevent recruitment to telomeres, instead resulting in the sequestration of telomerase in Cajal bodies.
Tables
Telomerase TEN domain mutant activity, processivity, and RAP stimulation by wild-type TPP1
| TEN domain mutant | Activity % of WT* | Processivity % of WT | RAP stimulation by PT† % of WT |
|---|---|---|---|
| R72E | 48 ± 3 | 99 ± 1 | 91 ± 2 |
| K78A | 19 ± 2 | 101 ± 7 | 88 ± 2 |
| K78E | 92 ± 1 | 100 ± 1 | 68 ± 5 |
| R87E;R91E;K94E | 74 ± 6 | 75 ± 0.3 | 61 ± 4 |
| R120E | 99 | 96 | 55 |
| K78E;R120E | 82 ± 18 | 87 ± 3 | 38 ± 2 |
| R132E | 73 ± 2 | 92 ± 3 | 24 ± 2 |
| R132E;K78E | 63 ± 10 | 94 ± 8 | 18 ± 1 |
| R142E | 97 ± 7 | 111 ± 10 | 103 ± 4 |
| R143E | 16 ± 12 | 81 ± 3 | N.D.‡ |
| R142E;R143E | 4 ± 1 | N.D.‡ | N.D.‡ |
| V144M | 48 ± 6 | 95 ± 5 | 44 ± 7 |
-
*
Percentage of wild-type telomerase activity, processivity, or RAP stimulation. Activity values normalized to hTERT levels, loading control, ± standard deviation for 2 or more replicates.
-
†
Repeat addition processivity (RAP) stimulation upon addition of WT POT1-TPP1, values relative to WT telomerase with WT POT1-TPP1 (i.e. RAP stimulation by PT % of WT = ((WT PT RAP stimulation of telomerase mutant)/(WT PT RAP stimulation WT telomerase))*100).
-
‡
Not determined (N.D.) due to low telomerase activity.





