The prenyltransferase UBIAD1 is the target of geranylgeraniol in degradation of HMG CoA reductase
Figures
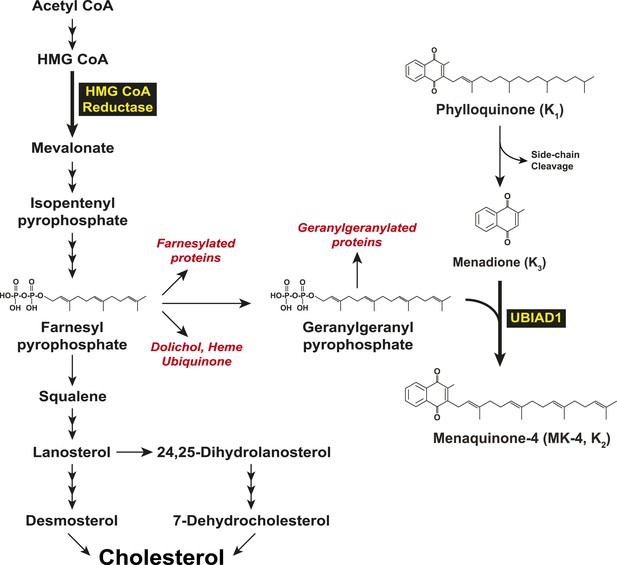
Biosynthesis of cholesterol and menaquinone-4 (MK-4, vitamin K2) in mammalian cells.
https://doi.org/10.7554/eLife.05560.003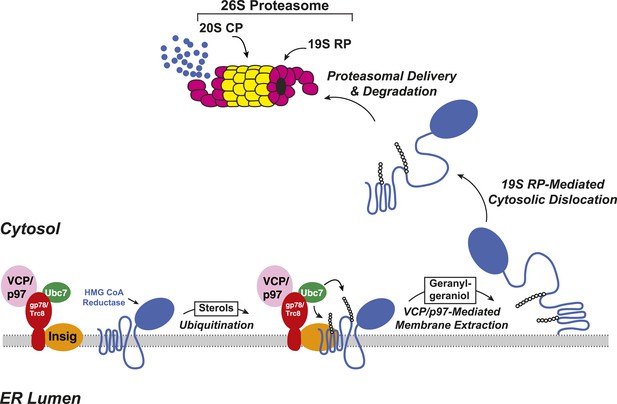
Insig-mediated, sterol-accelerated degradation of HMG CoA reductase in mammalian cells.
https://doi.org/10.7554/eLife.05560.004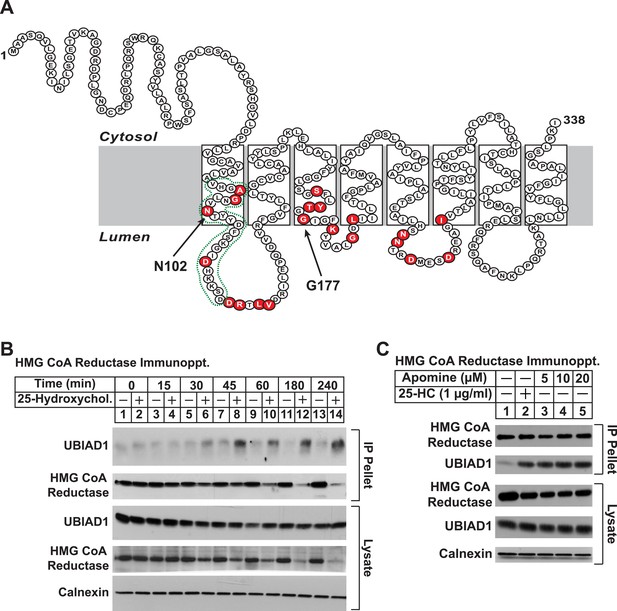
Identification of UBIAD1 as an associated protein of HMG CoA reductase.
(A) Amino acid sequence and predicted topology of human UBIAD1. Amino acid residues mutated in Schnyder corneal dystrophy (SCD) families are shaded in red; asparagine-102 (N102) and glycine-177 (G177), the two most frequently altered UBIAD1 residues in SCD, are indicated by arrows. The predicted active site in the prenyltransferase domain of UBIAD1 is indicated by the green dotted line. (B and C) SV-589 cells were set up for experiments on day 0 at a density of 2 × 105 cells per 100-mm dish in medium A supplemented with 10% FCS. On day 3, cells were switched to medium A containing 10% NC-LPPS, 10 µM sodium compactin, and 50 µM sodium mevalonate to deplete sterols. (B) After 16 hr at 37°C, the cells received sterol-depleting medium in the absence or presence of 1 µg/ml 25-HC and were further incubated for the indicated period of time 37°C. Cells were then harvested, lysed in PBS containing 1% digitonin, and the resulting lysates were subjected to immunoprecipitation with polyclonal anti-reductase antibodies as described in ‘Materials and methods’. Aliquots of precipitated material (IP Pellet) and lysates were subjected to SDS-PAGE and immunoblot analysis was carried out with IgG-H8 (against UBIAD1), IgG-A9 (against reductase), IgG-17H1 (against Insig-1), and anti-calnexin IgG. (C) Following sterol depletion, cells were incubated for 45 min at 37°C in sterol-depleting medium containing the indicated concentration of Apomine. Cells were then harvested, lysed, and immunoprecipitated with polyclonal anti-reductase; aliquots of precipitated material and lysates were analyzed by SDS-PAGE, followed by immunoblot as described in (B).
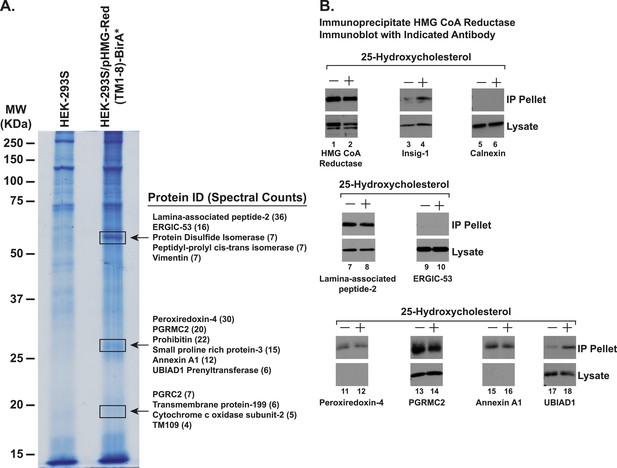
Identification of proteins associated with HMG-Red(TM1-8)-BirA*.
(A) HEK-293S/pHMG-Red(TM1-8)-BirA* cells were set up on day 0, depleted of sterols on day 3, harvested on day 4 for lysis and affinity purification using streptavidin-coupled beads as described in ‘Materials and methods’. Precipitated proteins were subjected to SDS-PAGE and the gel was subjected to staining with colloidal blue. Three segments of the gel (indicated by boxes) were excised and the identities of the proteins were determined by tandem mass spectrometry. The spectral count for the most abundant proteins identified in each segment is indicated in parentheses. (B) SV-589 cells were set up on day 0, depleted of sterols on day 3, and subjected to treatment in the absence or presence of 25-HC (1 µg/ml) for 45 min at 37°C on day 4 as described in the legend to Figure 3. Cells were then harvested, lysed in PBS containing 1% digitonin, and the resulting lysates were subjected to immunoprecipitation with polyclonal anti-reductase antibodies as described in ‘Materials and methods’. Aliquots of precipitated material (IP Pellet) and lysates were subjected to SDS-PAGE and immunoblot analysis was carried out with antibodies against the indicated protein.
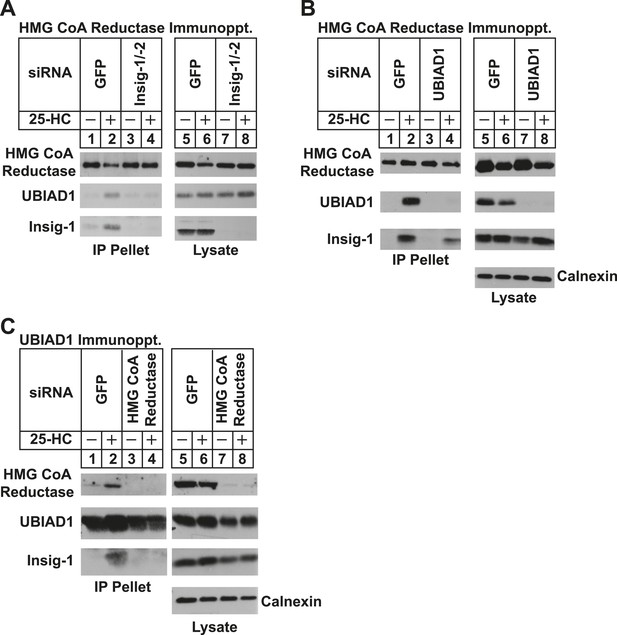
Specificity of sterol-dependent UBIAD1-HMG CoA reductase association.
SV-589 cells were set up on day 0 at 1 × 105 cells per 100-mm dish in medium A containing 10% FCS. On day 3, cells were transfected with siRNAs targeting mRNAs encoding GFP, Insig-1 and Insig-2, UBIAD1, or reductase as indicated and described in ‘Materials and methods’. Cells transfected with siRNA duplexes against reductase received 200 mM mevalonate to provide essential nonsterol isoprenoids. On day 4, cells were depleted of sterols through incubation for 16 hr at 37°C in medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate. The cells then received identical medium in the absence or presence of 1 µg/ml 25-HC. After 45 min at 37°C, cells were harvested, lysed, and immunoprecipitated with polyclonal antibodies against either reductase (A and B) or UBIAD1 (C). The resulting precipitated material and lysates were subjected to SDS-PAGE and immunoblot analysis with IgG-A9 (against reductase), IgG-H8 (against UBIAD1), IgG-17H1 (against Insig-1), and anti-calnexin IgG.
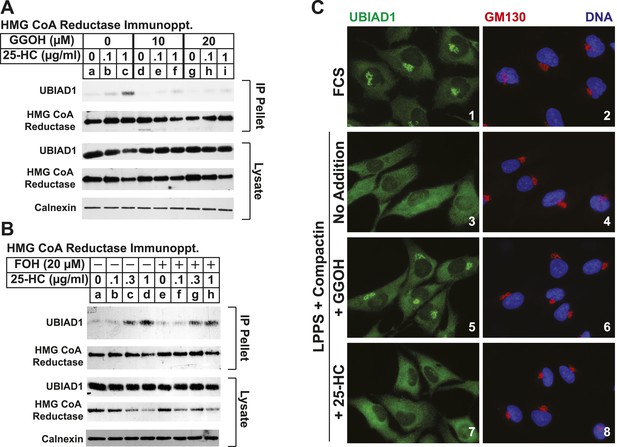
The nonsterol isoprenoid geranylgeraniol inhibits sterol-induced binding of UBIAD1 to HMG CoA reductase and promotes its translocation to the Golgi.
(A and B) SV-589 cells were set up for experiments on day 0 and depleted of sterols on day 3 as described in the legend to Figure 3. Following sterol-depletion, cells received medium A containing 10% NC-LPPS, 10 µM compactin, 50 µM mevalonate with the indicated concentration of 25-HC in the absence or presence of 10 or 20 µM geranylgeraniol (A) or 20 µM farnesol (B). Following incubation for 45 min at 37°C, cells were harvested, lysed, and immunoprecipitated with polyclonal antibodies against reductase. Aliquots of the resulting immunoprecipitates and lysates were subjected to immunoblot analysis with IgG-A9 (against reductase), IgG-H8 (against UBIAD1), IgG-17H1 (against Insig-1), and anti-calnexin IgG. (C) SV-589 cells were set up on day 0 at 7.5 × 104 cells/well of six-well plates with glass coverslips in medium A containing 10% FCS. On day 1, the cells were switched to identical medium or medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate as indicated. Following incubation for 16 hr at 37°C, the cells were treated in the absence or presence of 30 µM geranylgeraniol (GGOH) or 1 µg/ml 25-HC for an additional 4 hr at 37°C. The cells were subsequently fixed for microscopy as described in ‘Materials and methods’.
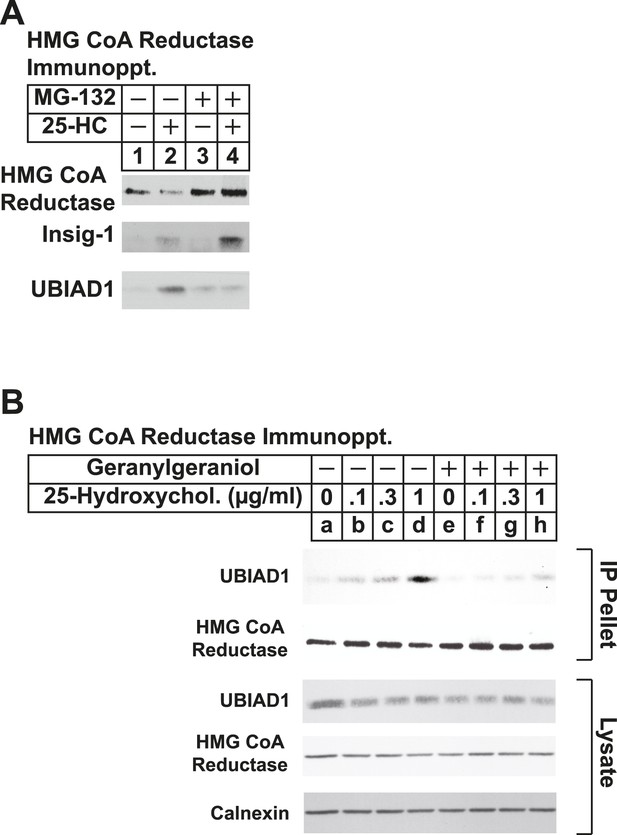
The proteasome inhibitor MG-132 and geranylgeraniol inhibit sterol-induced binding of UBIAD1 to HMG CoA reductase.
SV-589 cells were set up for experiments on day 0 and depleted of sterols on day 3 as described in the legend to Figure 3. Following sterol-depletion, cells received medium A containing 10% NC-LPPS, 10 µM compactin, 50 µM mevalonate in the absence or presence of 10 µM MG-132 for 1 hr at 37°C, followed by treatment in the absence or presence of 1 µg/ml 25-HC (A) or the indicated concentration of 25-HC in the absence or presence of 15 µM geranylgeraniol (B). Following incubation for 45 min at 37°C, cells were harvested, lysed, and immunoprecipitated with polyclonal antibodies against reductase. Aliquots of the resulting immunoprecipitates and lysates were subjected to immunoblot analysis with IgG-A9 (against reductase), IgG-H8 (against UBIAD1), IgG-17H1 (against Insig-1), and anti-calnexin IgG.
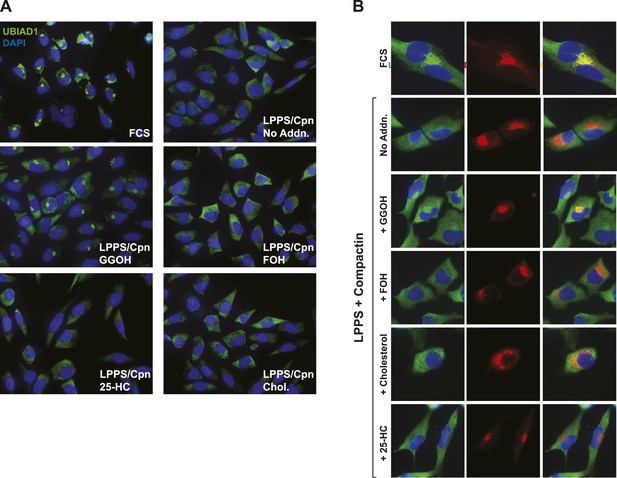
Geranylgeraniol, but not 25-HC, FOH, or cholesterol, stimulates translocation of endogenous UBIAD1 to the Golgi in cells deprived of sterol and nonsterol isoprenoids.
(A) SV-589 cells were set up on day 0 at 7.5 × 104 cells/well of six-well plates with glass coverslips in medium A containing 10% FCS. On day 1, the cells were switched to identical medium or medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate as indicated. Following incubation for 16 hr at 37°C, the cells were treated in the absence or presence of 30 µM geranylgeraniol (GGOH), 30 µM farnesol (FOH), 1 µg/ml 25-HC, or 10 µg/ml cholesterol for an additional 4 hr at 37°C. The cells were subsequently fixed and subjected to immunostaining as described in the legend to Figure 5. Coverslips were mounted using Fluoromount G (Electron Microscopy Sciences, Hatfield, PA). Images were obtained with a Zeiss Axio Observer Epifluorescence microscope using a 63× oil Plan-Apochromat objective and Ziess Axiocam color digital camera in black and white mode. For the purpose of presentation, brightness levels were adjusted across the entire image using ImageJ software (National Institution of Health, USA). (B) SV-589 cells were set up on day 0 at 3 × 104 cells/well of a twelve-well plate with glass coverslips. On day 1, the cells were transfected using FuGENE6 with 200 ng DsRed-Golgi (Clontech Laboratories, Inc., Mountain View, CA); the total amount of DNA/well was adjusted to 500 ng by the addition of empty pcDNA3.1 vector. 4 hr after transfection, cells received a direct addition of medium A containing 10% FCS or 10% NC-LPPS supplemented with 10 µM compactin and 50 µM mevalonate (final concentrations) as indicated. Following incubation for 16 hr at 37°C, cells were treated for 4 hr in the absence or presence of 30 µM geranylgeraniol (GGOH), 30 µM farnesol (FOH), 1 µg/ml 25-HC, or 10 µg/ml cholesterol. Cells were subsequently fixed and subjected to immunostaining and imaging as described in (A). Shown are cropped images representing a 64 × 64 micron-portion of the original images 219 × 174 microns in size.
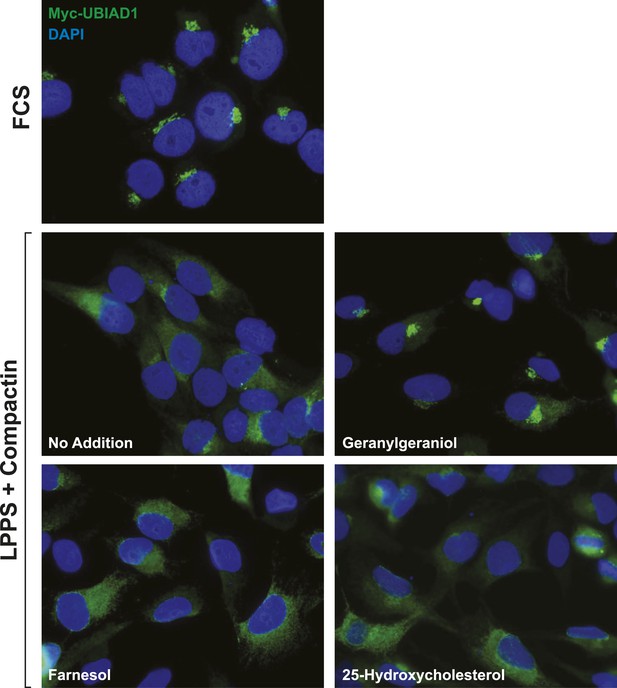
Geranylgeraniol, but not 25-HC or farnesol, stimulates translocation of transfected UBIAD1 to the Golgi in cells deprived of sterol and nonsterol isoprenoids.
SV-589/pMyc-UBIAD1 cells, a line of SV-589 cells that stably express Myc-UBIAD1, were generated as follows. SV-589 cells were set up on day 0 at a density of 7 × 105 cells per 100-mm dish in medium A supplemented with 10% FCS. On day 1, cells were transfected with 2 µg/dish of pCMV-Myc-UBIAD1 using FuGENE6 transfection reagent as described in ‘Materials and methods’. Following incubation for 16 hr at 37°C, cells were switched to medium A supplemented with 10% FCS and 700 µg/ml G418. Fresh medium as added every 2–3 days until colonies formed after 2 weeks. Individual colonies were isolated using cloning cylinders, and expression of Myc-UBIAD1 was determined by immunoblot analysis. Select colonies were expanded and then further purified by serial dilution in 96-well plates. Individual clones were screened by immunofluorescense using IgG-9E10 against the Myc epitope. For experiments, SV-589/pMyc-UBIAD1 cells were set up on day 0 at 7.5 × 104 cells/well of six-well plates with glass coverslips in medium A containing 10% FCS. On day 1, the cells were switched to identical medium or medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate as indicated. Following incubation for 16 hr at 37°C, the cells were treated in the absence or presence of 30 µM geranylgeraniol, 30 µM farnesol, or 1 µg/ml 25-HC for an additional 4 hr at 37°C. The cells were subsequently fixed and subjected to immunostaining and analysis as described in the legend to Figure 5—figure supplement 2.
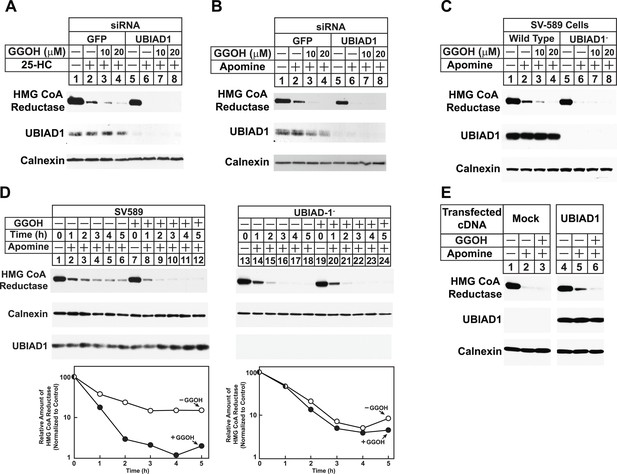
RNA interference-mediated knockdown or CRISPR/Cas9-mediated knockout of UBIAD1 alleviates geranylgeraniol requirement in sterol-accelerated degradation of HMG CoA reductase.
(A and B) SV-589 cells were set up for experiments on day 0, transfected with the indicated siRNA duplexes on day 3, and depleted of sterols as described in the legend to Figure 4. The sterol-depleted cells were then treated with medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate in the absence or presence of 1 µg/ml 25-HC and geranylgeraniol (GGOH) as indicated. Following incubation for 4 hr at 37°C, cells were harvested for subcellular fractionation. Aliquots of resulting membrane fractions (20 µg protein/lane) were subjected to SDS-PAGE and immunoblot analysis was carried out with IgG-A9 (against reductase), IgG-H8 (against UBIAD1), and anti-calnexin IgG. (C–E) SV-589, UBIAD1−, UBIAD1−/pCDNA3.1, and UBIAD1−/pMyc-UBIAD1 cells were set up for experiments on day 0 at 3.5 × 105 cells per 60-mm dish in medium A containing 10% FCS. On day 1, cells were switched to medium A supplemented with 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate. Following incubation for 16 hr at 37°C, cells received the identical medium in the absence or presence of 10 µM Apomine and the indicated concentration (C) or 10 µM (D and E) of geranylgeraniol (GGOH). After the indicated period of time (D) or 5 hr (C and E) at 37°C, cells were subjected to subcellular fractionation and membrane fractions (12 µg protein/lane) were analyzed by immunoblot as described in (A).
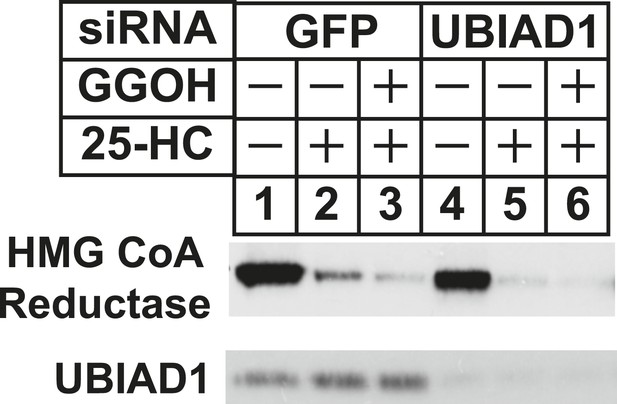
RNA interference-mediated knockdown of UBAD1 alleviates requirement for geranylgeraniol in sterol-accelerated reductase degradation.
SV-589 cells were set up for experiments on day 0, transfected with the indicated siRNA duplexes on day 3, and depleted of sterols as described in the legend to Figure 3. Notably, the siRNA duplex targeting UBIAD1 (5′-UCUUGGAGCCGCAGGAUGUUU-3′, Dharmacon/ThermoScientfic) was distinct from that used in Figures 3, 6. The sterol-depleted cells were then treated with medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate in the absence or presence of 1 µg/ml 25-HC and 20 µM geranylgeraniol (GGOH). Following incubation for 4 hr at 37°C, cells were harvested for subcellular fractionation. Aliquots of resulting membrane fractions (20 µg protein/lane) were subjected to SDS-PAGE and immunoblot analysis was carried out with IgG-A9 (against reductase) and IgG-H8 (against UBIAD1).
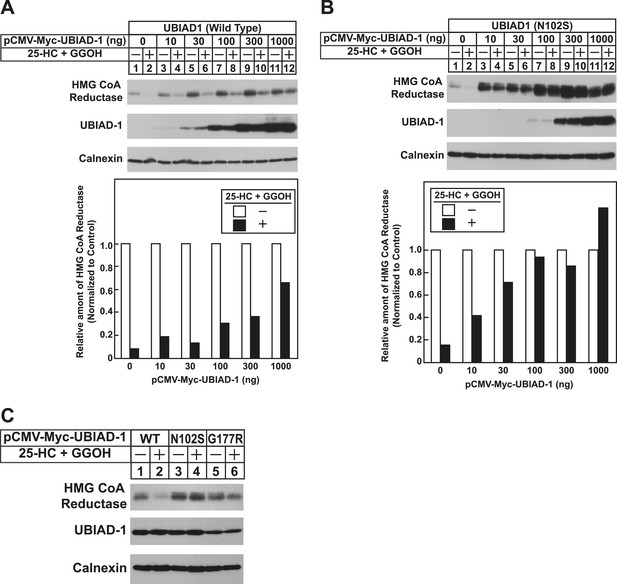
The Schnyder corneal dystrophy (SCD)-associated N102S and G177R mutants of UBIAD1 block sterol-accelerated ERAD of HMG CoA reductase.
SV-589 cells were set up for experiments on day 0 at 4 × 105 cells per 60-mm dish in medium A containing 10% FCS. On day 1, cells were transfected with 3 µg/dish of pCMV-HMG-Red(TM1-8)-T7 in the absence or presence of the indicated concentration of pCMV-Myc-UBIAD1 (WT or N102S) (A and B) or 3 µg/dish of pCMV-HMG-Red(TM1-8)-T7 together with 30 ng of pCMV-Myc-UBIAD1 (WT, N102S, or G177R) (C) as described in ‘Materials and methods’. 4 hr after transfection, cells received a direct addition of medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate (final concentrations). Following incubation for 16 hr at 37°C, cells were treated with identical medium in the absence or presence of 1 µg/ml 25-HC plus 20 µM geranylgeraniol (GGOH) as indicated. After 4 hr at 37°C, cells were harvested and subjected to subcellular fractionation. Aliquots of resulting membrane fractions were then subjected to SDS-PAGE and immunoblot analysis was carried out with anti-T7 IgG (against reductase), IgG-9E10 (against UBIAD1 and Insgi-1), and anti-calnexin IgG. Proteins corresponding to reductase in (A and B) were quantified using ImageJ software. The intensities of these signals in the absence of 25-HC plus geranylgeraniol were arbitrarily set as 1.
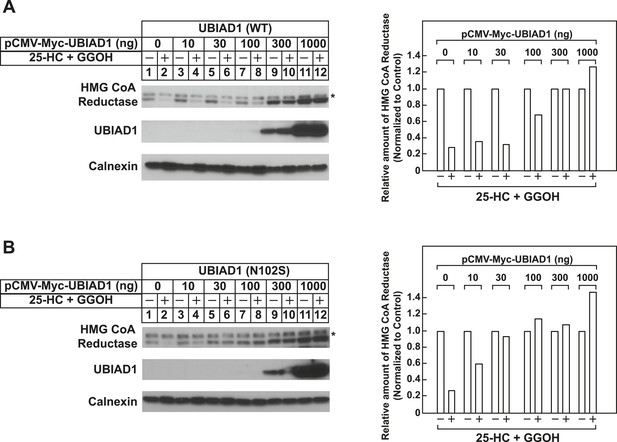
Schnyder corneal dystrophy (SCD)-associated N102S mutant of UBIAD1 blocks sterol-accelerated ERAD of full-length HMG CoA reductase.
SV-589 cells were set up for experiments on day 0 at 4 × 105 cells per 60-mm dish in medium A containing 10% FCS. On day 1, cells were transfected with 3 µg/dish of pCMV-HMG-Red-T7 in the absence or presence of the indicated concentration of wild type (A) or N102S (B) versions of pCMV-Myc-UBIAD1 as described in ‘Materials and methods’. 4 hr after transfection, cells received a direct addition of medium A containing 10% NC-LPPS, 10 µM compactin, and 50 µM mevalonate (final concentrations). Following incubation for 16 hr at 37°C, cells were treated with identical medium in the absence or presence of 1 µg/ml 25-HC plus 20 µM geranylgeraniol (GGOH) as indicated. After 4 hr at 37°C, cells were harvested and subjected to subcellular fractionation. Aliquots of resulting membrane fractions were then subjected to SDS-PAGE and immunoblot analysis was carried out with anti-T7 IgG (against reductase), IgG-9E10 (against UBIAD1 and Insgi-1), and anti-calnexin IgG. Asterisks denote a non-specific cross-reactive band. Proteins corresponding to reductase in (A and B) were quantified using ImageJ software. The intensities of these signals in the absence of 25-HC plus geranylgeraniol were arbitrarily set as 1.
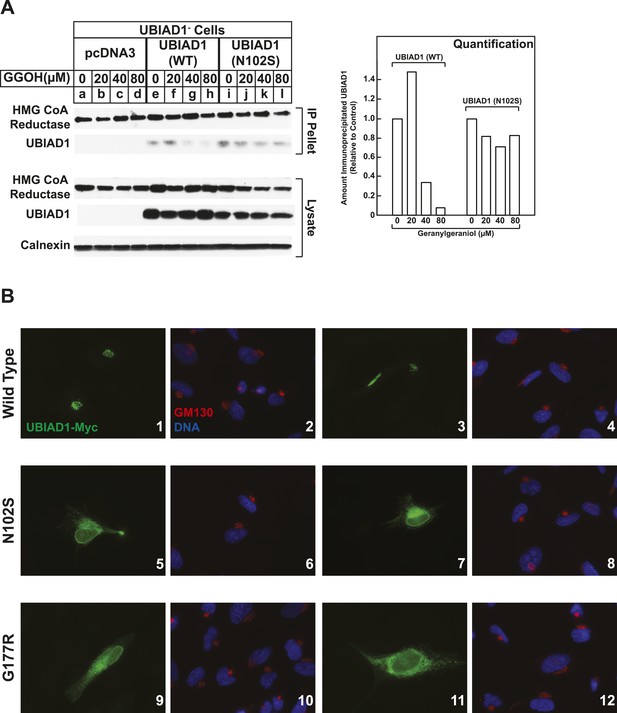
SCD-associated UBIAD1 mutant resists geranylgeraniol-mediated displacement from HMG CoA reductase and remains sequestered in ER membranes.
(A) UBIAD1−/pCDNA3.1, UBIAD1−/pMyc-UBIAD1 (WT), and UBIAD1−/pMyc-UBIAD1 (N102S) cells were set up for experiments on day 0 at a density of 4 × 105 cells per 60-mm dish in medium A containing 10% FCS. On day 3, cells were depleted of sterols as described in the legend to Figure 4. After 16 hr at 37°C, cells received the identical medium containing 1 µg/ml 25-HC in the absence or presence of the indicated concentration of geranylgeraniol. After 45 min at 37°C, cells were harvested, lysed, and immunoprecipitated with polyclonal anti-reductase antibodies. Aliquots of the precipitated material and the lysates were subjected to SDS-PAGE and immunoblot analysis was carried out with IgG-A9 (against reductase), IgG-H8 (against UBIAD1), and anti-calnexin IgG. Proteins corresponding to immunoprecipitated UBIAD1 were quantified using ImageJ software. The intensities of these signals in the absence of geranylgeraniol were arbitrarily set as 1. (B) SV-589 cells were set up on day 0 at 3 × 104 cells/well of a twelve-well plate with a glass coverslip in medium A containing 10% FCS. On day 1, the cells were transfected using FuGENE 6 with 50 ng of wild type (WT), N102S, or G177R versions of pCMV-Myc-UBIAD1; the total amount of DNA/well was adjusted to 500 ng by the addition of pcDNA3.1 vector. 4 hr after transfection, cells received a direct addition of medium A containing 10% FCS (final concentration). After 16 hr at 37°C, cells were fixed and analyzed by microscopy as described in ‘Materials and methods’.
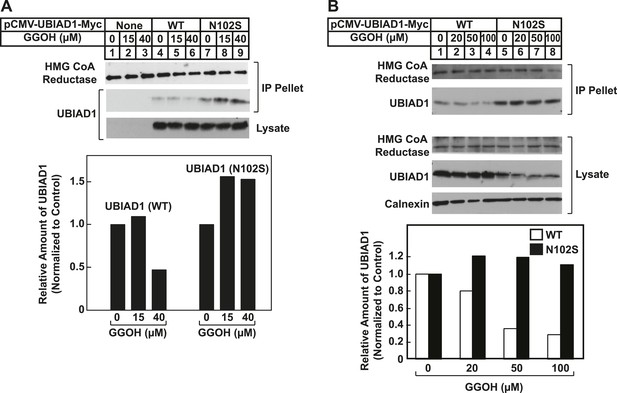
SCD-associated UBIAD1 (N102S) resists geranylgeraniol-mediated displacement from HMG CoA reductase in two independent experiments (A and B).
UBIAD1−/pCDNA3.1, UBIAD1−/pMyc-UBIAD1 (WT), and UBIAD1−/pMyc-UBIAD1 (N102S) cells were set up for experiments on day 0 at a density of 4 × 105 cells per 60-mm dish in medium A containing 10% FCS. On day 3, cells were depleted of sterols as described in the legend to Figure 4. After 16 hr at 37°C, cells received the identical medium containing 1 µg/ml 25-HC in the absence or presence of the indicated concentration of geranylgeraniol. After 45 min at 37°C, cells were harvested, lysed, and immunoprecipitated with polyclonal anti-reductase antibodies. Aliquots of the precipitated material and the lysates were subjected to SDS-PAGE and immunoblot analysis was carried out with IgG-A9 (against reductase), IgG-H8 (against UBIAD1), and anti-calnexin IgG. Proteins corresponding to immunoprecipitated UBIAD1 were quantified using ImageJ software. The intensities of these signals in the absence of geranylgeraniol were arbitrarily set as 1.
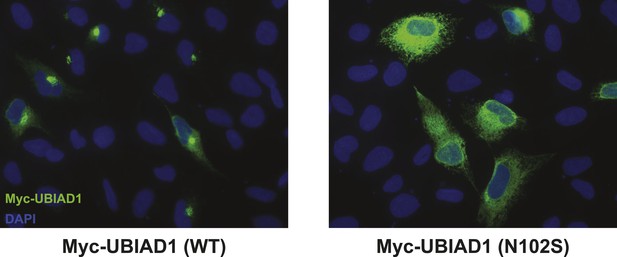
Subcellular localization of wild type and SCD-associated N102S UBIAD1 in transfected SV-589 cells.
SV-589 cells were set up for experiments on day 0, transfected on day 1 with pCMV-Myc-UBIAD1 (WT) or (N102S) in medium A containing 10% FCS, and subjected to immunostaining, followed by imaging as described in the legend to Figure 5—figure supplement 1.
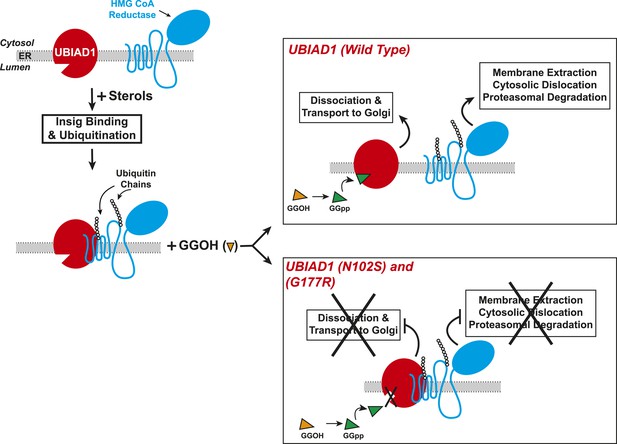
Proposed model for role of UBIAD1 in sterol-accelerated degradation of HMG CoA reductase.
In sterol-deprived cells, both reductase and UBIAD1 localize to membranes of the ER. The intracellular accumulation of sterols in ER membranes triggers binding of reductase to Insigs, resulting in its ubiquitination by Insig-associated ubiquitin ligases gp78 and Trc8 and association with UBIAD1. Geranylgeraniol becomes phosphorylated to produce geranylgeranyl pyrophosphate, which enhances reductase degradation by binding to UBIAD1, causing its displacement from reductase-Insig. This displacement allows for transport of UBIAD1 to the Golgi and membrane extraction, cytosolic dislocation, and proteasomal degradation of reductase. We postulate that the SCD-associated N102S or G177R mutations in UBIAD1 abrogate binding of geranylgeranyl pyrophosphate. As a result, UBIAD1 (N102S) and (G177R) do not translocate to the Golgi and remain associated with reductase in the ER, thereby blocking its membrane extraction, cytosolic dislocation, and proteasomal degradation.





