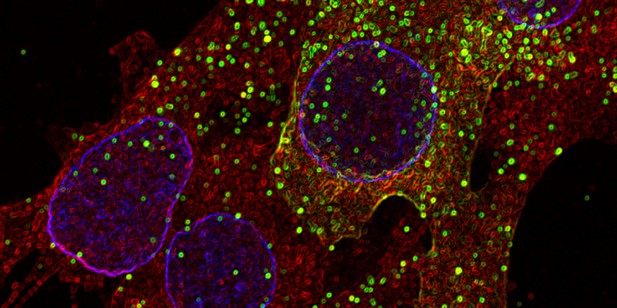
HIV-1 (green) uses a protein called Vpu to suppress immune responses of cells (cell nuclei in blue) by blocking an important regulator of the immune system (NF-κB, red). Image credit: Kristina Hopfensperger, Konstantin Sparrer (CC-BY 4.0)
The Human Immunodeficiency Virus (or HIV for short) has infected more than 70 million people worldwide. Although effective therapies exist to prevent the replication of the virus and the development to AIDS, there is no cure or vaccine, and the virus still spreads efficiently in human populations, infecting about 1.8 million new people every year.
The unfortunate success of HIV can in part be explained by several viral proteins that trick our immune system and enable the virus to persist at high levels in the human body. For example, an HIV protein called viral protein U (Vpu) prevents infected cells from producing alarm signals such as interferons, which usually help healthy, uninfected cells to defend themselves against viruses. However, the extent to which Vpu interferes with interferons and other proteins involved in immune responses has remained unclear.
To address this question, Langer, Hammer, Hopfensperger et al. compared how different variants of HIV affect immune responses in human cells. The experiments showed that cells infected with HIV variants lacking Vpu released larger amounts of interferons and other cellular proteins that are involved in immune responses compared to HIV variants with Vpu. Further experiments showed that Vpu works by inhibiting the activation of a protein called NF-κB, which usually switches on genes that encode interferons and many other proteins involved in immune responses.
These findings demonstrate that Vpu has a broader impact on the human immune response than previously thought. In order to multiply efficiently, HIV initially requires the NF-κB protein to be active. Therefore, when NF-κB is inactive, HIV may adopt a dormant state that prevents current antiviral drug treatments from eradicating the virus in the human body. In the future, developing new drugs that can activate dormant HIV particles may therefore have the potential to help cure HIV infections.




