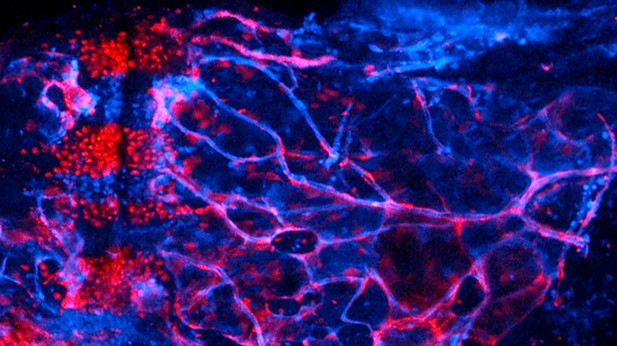
As cartilage cells (in red) migrate into the marrow cavity (on the right), they transition into bone, fat, and potential stem cells within adult bones. Image credit: Dion Giovannone (CC BY 4.0)
Our adult bones are made of a fatty tissue, called marrow, wrapped inside a hard outer layer produced by bone cells. They may appear stiff and unyielding, but our bones are actually dynamic structures. Early in life, most bones start as small ‘templates’ made of another, flexible tissue called cartilage. As the templates grow into adult bones, the cartilage is gradually replaced by bone and fat, but this process is still poorly understood. For example, it is not clear whether cartilage cells simply die and make way for new cells, or instead if they turn into bone and fat cells. To investigate this question, Giovannone, Paul et al. set out to follow the fate of early cartilage cells in zebrafish, and to compare this with what happens in mammals. Zebrafish were chosen because their skeleton and ours develop in similar ways; yet, these animals are much easier to study, in particular because their embryos are transparent.
Young cartilage cells were ‘tagged’ with a long-lasting fluorescent protein in genetically engineered zebrafish embryos, and then followed over time. As the embryos started to form bones, the cartilage cells gave rise to bone cells, fat cells, and also potentially adult stem cells within the marrow, which can become other types of cells. This process required a protein called Mmp9, which also helps shape bone development in other organisms, including humans.
Defects in how early cartilage templates morph into bone and fat may contribute to dwarfism and other severe conditions. Fully grasping the molecular mechanisms that preside over this complex transition may one day help design drugs to treat skeletal disorders.




