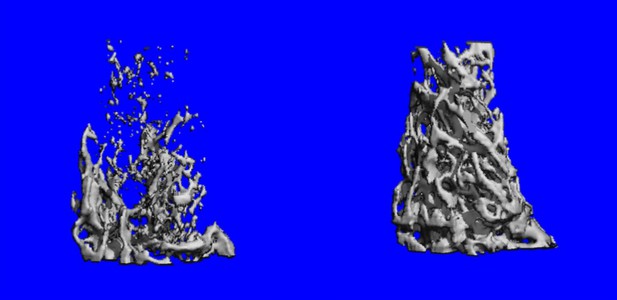
Bone density is greater when the Rgs12 gene is removed (right). Image credit: Ng, Li et al. (CC BY 4.0)
Human bodies change with age, and the skeleton is among the parts of the body most visibly affected. This is because bone tissue tends to decrease as the skeleton gets older. For example, people often get shorter as they get older, mostly because they lose bone mass in areas of the skeleton that support posture. Severe bone loss can also lead to osteoporosis, a debilitating condition where bones become brittle and fracture easily.
Human skeletons contain cells, called osteoclasts, which break down bone tissue. Osteoclasts normally cooperate with other cells that add new bone, which helps maintain the balance between ‘bone-eating’ and ‘bone-building’ responsible for sculpting a healthy skeleton. This balance is disrupted during old age when the body starts producing too many ‘hyperactive’ osteoclasts, and bone formation cannot keep up with bone loss.
Reactive oxygen species (ROS) are unstable, potentially toxic molecules that have been linked with diseases of aging. Recent research has shown that low amounts of ROS can also drive the formation of new osteoclasts. Ng, Li et al. therefore wanted to determine how exactly ROS did this – specifically, whether ROS works together with the cell signaling mechanisms involved in bone loss controlled by a gene called Rgs12.
Initial experiments, using genetically altered mice, showed that removing Rgs12 from immature osteoclasts was enough to stop them from maturing. The bones of these mice were also stronger and thicker than usual. In contrast, forcing osteoclasts to produce large amounts of the protein encoded by Rgs12 heightened their bone-eating ability.
Analysis of the proteins made by cells without Rgs12 revealed that the cells had turned on the Nrf2 gene, a molecular ‘master switch’ that helps produce the enzymes capable of counteracting ROS (termed antioxidants). These cells therefore contained abnormally high amounts of antioxidants and low levels of ROS. However, osteoclasts where the Rgs12 gene was present were able to generate ROS by switching off the Nrf2 gene, and were thus able to reach maturity.
These results shed new light on the molecular signals that direct the development and activity of osteoclasts. In the future, a better understanding of these mechanisms could help us prevent them going wrong during aging, or even lead to better therapies for osteoporosis and other skeletal disorders.




