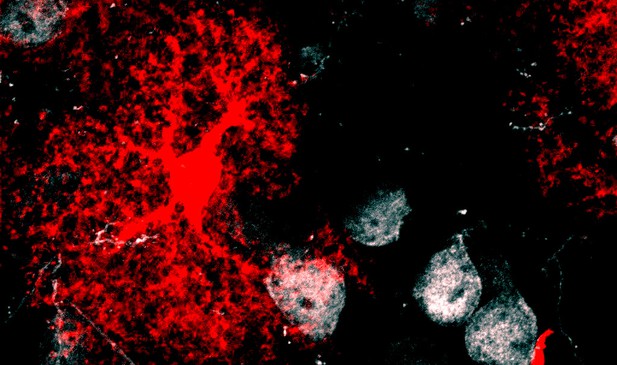
Image of an astrocyte (red) surrounding the body of a neuron (white). Image credit: Steven Hill (CC BY 4.0)
A central system of neurons in the spinal cord and brain coordinate most of our body’s actions, ranging from regulating our heart rate to controlling our movement and thoughts. As the brain develops, neurons form specialized contacts with one another known as synapses. If the number of synapses is not properly regulated this can disrupt communication between the neurons, leading to diseases like schizophrenia and autism.
As the brain develops, it first forms an excess of synapses and later eliminates unnecessary or weak connections. Various factors, such gene expression or a neuron’s level of activity, regulate this turnover process. However, neurons cannot do this alone, and rely on other types of cells to help regulate their behavior. In the central nervous system, for example, a cell called an astrocyte is known to support the formation and activity of synapses. Now, Hill and Blaeser et al. show that astrocytes also exert influence over synaptic turnover during development, leading to long lasting changes in the number of synapses.
Hill, Blaeser et al. revealed that disrupting activity of the signaling pathway known as Sonic hedgehog, or Shh for short, in the astrocytes of mice led to disordered synaptic connections. Notably, neurons produce Shh, suggesting that neurons use this signaling pathway to communicate to specific astrocyte partners. Further experiments showed that reducing astrocyte’s ability to respond to Shh impaired synaptic turnover as the brain developed, leading to an overabundance of synapses. Importantly, these effects were only found to influence neuron populations associated with astrocytes that actively use Shh signaling. This suggests that distinct populations of neurons and astrocytes interact in specialized ways to build the connections within the nervous system.
To address how astrocytes use Shh signaling to regulate synaptic turnover, Hill, Blaeser et al. examined gene expression changes in astrocytes that lack Shh signaling. Astrocytes with a reduced capacity to respond to Shh were found to have lower levels of a protein responsible for transporting potassium ions into and out of the cell. This impairs astrocyte’s ability to regulate neuronal activity, which may lead to a failure in eliminating unnecessary synapses.
Understanding how synapses are controlled and organized by astrocytes could help identify new ways to treat diseases of the developing nervous system. However, further studies would be needed to improve our understanding of how this process works.




