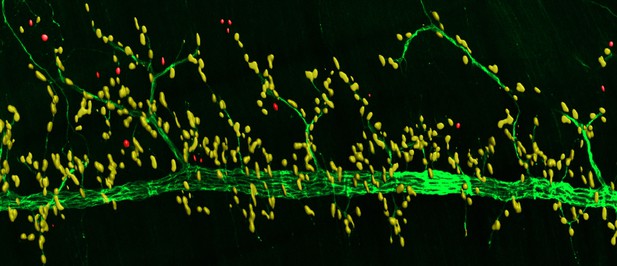
When nerve cells (green) innervate muscle cells, a molecule called MT1-MMP helps recruit spontaneously formed clusters of acetylcholine receptors (red) to the communication site (yellow) between these two cells. Image credit: Chan et al. (CC BY 4.0)
Voluntary movement relies on skeletal muscle cells and nerve cells being able to communicate with one another. This communication occurs at a specialized region called the neuromuscular junction, or NMJ for short. These junctions are surrounded by a meshwork of proteins, known as the matrix, which structurally supports the nerve and muscle cells.
Muscle cells contain proteins called acetylcholine receptors on their cell surface. When these receptors cluster together at the NMJ, this allows nerve cells to communicate with the muscle cell and tell the muscle to contract. However, these clusters can also form spontaneously without the help of nerve cells at regions away from the communication site. Alongside these spontaneous clusters of acetylcholine receptors are dynamic actin-enriched structures. These structures are responsible for releasing enzymes that digest the surrounding matrix and are commonly found in migrating cells. But as skeletal muscle cells do not migrate, it remained unclear what purpose these structures serve at the NMJ.
Now, Chan et al. have used advanced microscopy techniques to show how these actin-enriched structures can help acetylcholine receptors cluster together at the site of communication between the nerve and muscle cells. The experiments showed that these structures direct a molecule called MT1-MMP to the muscle surface. This molecule then clears the surrounding matrix so that signals sent from the nerve can be effectively deposited at the narrow space between these two cells. When the muscle cells receive this initiating signal, acetylcholine receptors are recruited from the spontaneously formed clusters to the communication site, allowing the muscle to contract. When MT1-MMP was experimentally eliminated in mice, this disrupted the recruitment of acetylcholine receptors to the NMJ.
Overall, these experiments help researchers understand how clearing the matrix between nerve and muscle cells contributes to the deposition of factors that build the communication site at developing NMJs. In the future this might help develop treatments for movement disorders caused by abnormalities that affect the clearing of matrix proteins in these junctions.




