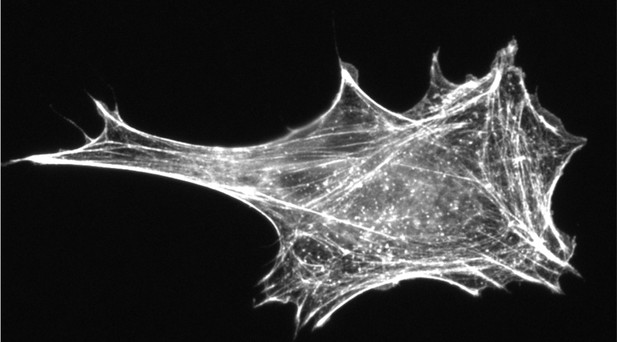
The complex web of actin filaments in a cell. Image credit: Vedula et al. (CC BY 4.0)
Most mammalian cells make both β- and γ-actin, two proteins which shape the cell’s internal skeleton and its ability to migrate. The molecules share over 99% of their sequence, yet they play distinct roles. In fact, deleting the β-actin gene in mice causes death in the womb, while the animals can survive with comparatively milder issues without their γ-actin gene. How two similar proteins can have such different biological roles is a long-standing mystery.
A closer look could hold some clues: β- and γ-actin may contain the same blocks (or amino acids), but the genetic sequences that encode these proteins differ by about 13%. This is because different units of genetic information – known as synonymous codons – can encode the same amino acid. These ‘silent substitutions’ have no effect on the sequence of the proteins, yet a cell reads synonymous codons (and therefore produces proteins) at different speeds.
To find out the impact of silent substitutions, Vedula et al. swapped the codons for the two proteins, forcing mouse cells to produce β-actin using γ-actin codons, and vice versa. Cells with non-manipulated γ-actin and those with β-actin made using γ-actin codons could move much faster than cells with β-actin. This suggested that silent substitutions were indeed affecting the role of the protein.
Vedula et al. found that cells read γ-codons – and therefore made γ-actin – much more slowly than β-codons: this also affected how quickly the protein could be dispatched where it was needed in the cell. Slower production meant that bundles of γ-actin were shorter, which allowed cells to move faster by providing a weaker anchoring system. Overall, this work provides new links between silent substitutions and protein behavior, a relatively new research area which is likely to shed light on other protein families.




