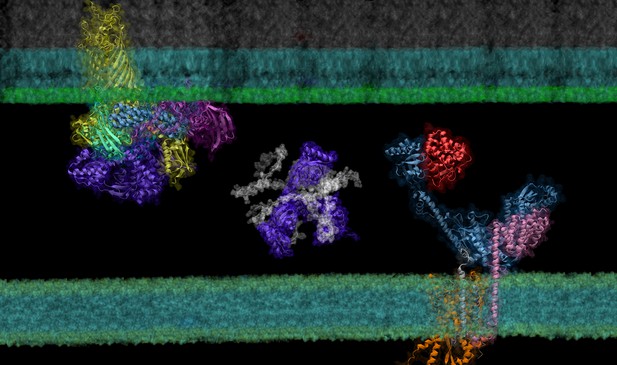
In certain types of bacteria proteins required for survival move from the inner cell membrane (bottom green line) to the outer membrane (top green line) with the help of specialised transport proteins. Proteins are visualised here using their predicted molecular structure. Image credit: Mu Gao (CC BY 4.0)
All living cells are contained within a fatty cell membrane that allows water and only certain other molecules to pass through with ease. Bacteria only consist of a single cell, making their membrane the only interface with the surrounding environment. Gram-negative bacteria – which include Escherichia coli, a bacterium found in the gut of all humans – have an extra layer of protection, the ‘outer membrane’. Proteins in this membrane are called ‘outer membrane proteins’ or OMPs and allow nutrients to enter the cell. But OMPs, which are made inside the cell, need to be transported to the outer membrane and folded correctly before they can perform their role. This multistep process, which involves interactions between many different proteins, is not fully understood.
The journey of an OMP from the center of the cell where it is made to the outer membrane is complicated. First, the OMP needs to pass through the cell’s inner membrane. To do this, it must interact with ‘channel proteins’ in the inner membrane that feed the OMP into the space between the two membranes, known as the bacterial envelope. This step requires the OMP to be unfolded. Once in the bacterial envelope the OMP interacts with proteins that help it fold correctly and integrate into the outer membrane.
The interactions between proteins in the bacterial envelope are short-lived, making them difficult to study using lab-based experiments. An alternative approach is predicting a protein’s structure from its amino acid sequence which is a difficult computational problem to solve. However, in 2020 developers behind the AlphaFold2, a deep learning program, were able to use a set of equations organized in a ‘neural network’ that can ‘learn’ from a library of known protein structures to predict unknown structures with high accuracy. Gao et al. used AF2Complex, a tool based AlphaFold2, tailored to predicting interactions between proteins, to investigate what interactions OMPs could be involved with on their way to the outer membrane.
With the help of a supercomputer at the Oakridge National Laboratory, Gao et al. screened nearly 1,500 E. coli proteins within the bacterial envelope to see how they might interact with OMPs. The screen identified previously unknown interactions between proteins that suggest that the formation of the bacterial outer membrane and the integration of proteins into it involve protein complexes and molecular mechanisms that have not yet been characterized. Additionally, the screen also identified interactions that had been previously described, confirming that the deep learning approach can correctly capture real interactions.
Overall, Gao et al.’s work inspires new hypotheses about the mechanisms through which OMPs are transported to the outer membrane, although further work will be needed to confirm the roles of protein interactions predicted by the computational model experimentally. Furthermore, the ability to design experiments based on computational predictions is exciting. If confirmed, the new protein interactions could help scientists better understand OMP transport, which is essential for bacterial biology. In the future, this could lead to the discovery of new targets for antibiotic drugs.




