Eph and Ephrin function in dispersal and epithelial insertion of pigmented immunocytes in sea urchin embryos
Figures
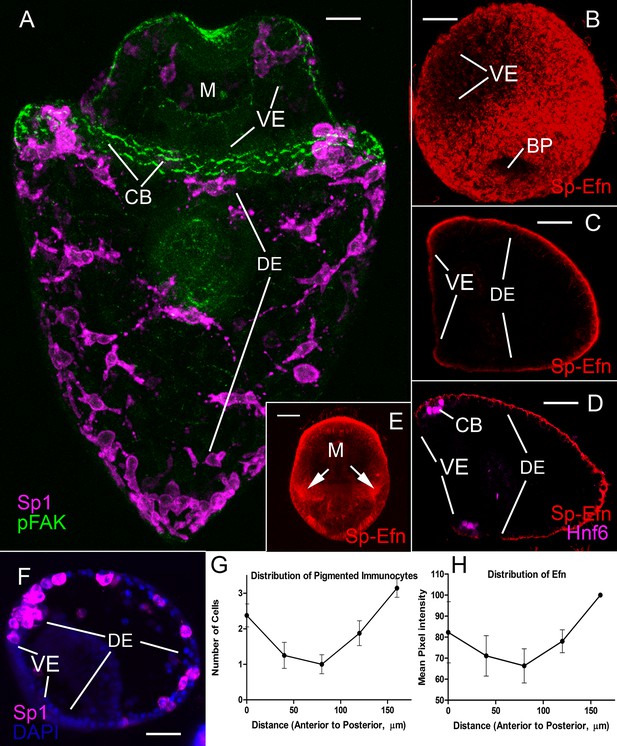
The distribution of pigmented immunocytes correlates with the abundance of Sp-Efn.
(A) Maximum intensity projection of confocal optical sections of an early pluteus larva prepared with Sp1, a pigmented immunocyte cell surface marker, and pFAK, a marker for apical junctions that are prominent in ciliary band cells (MeOH fixation). Pigmented immunocytes are scattered throughout the dorsal ectoderm (DE), which is the larval surface posterior to the ciliary band (CB). Immunocytes do not normally insert in the ventral ectoderm (VE), which is the larval surface surrounding the mouth (M) and encircled by the ciliary band. Immunocytes are most dense at the tips of larval arms, along the edge of the ciliary band, and the posterior tip of the larval body. Bar = 15 µm (B) Maximum intensity projection of confocal optical sections of a gastrula prepared with an antibody to Sp-Efn. The ligand is not expressed in the ventral ectoderm (VE). BP, blastopore (PFA fixation) Bar = 20 µm (C) Single sagittal optical section of a prism larva showing the expression of Sp-Efn only in the dorsal ectoderm and ciliary band and not in the ventral ectoderm (VE) (PEM fixation) Bar = 20 µm (D) Single sagittal optical section of an early pluteus larva showing the expression of Sp-Efn and the ciliary band marker, Hnf6. Sp-Efn expression is not uniform throughout the dorsal ectoderm, there is an apparent gradation of expression that is highest at the vertex of the larva. (PFA fixation). Bar = 20 µm (E) Maximum intensity projection of an early pluteus prepared with anti-Sp-Efn and oriented with the ventral surface foremost. Arrows indicate high levels of immunoreactivity where the postoral larval arms are situated. M, mouth (PEM fixation) Bar = 20 µm (F) Mid sagittal optical section of an early pluteus prepared with Sp1 to show the distribution of pigmented immunocytes (MeOh fixation). Bar = 20 µm (G) The distribution of pigmented immunocytes in the ectoderm between the animal pole domain and the vertex was determined from a set of mid-sagittal images from 6 embryos prepared with Sp1 (see Figure 1—figure supplement 1). The distance from the vertex of the larva along the surface to the animal pole domain was divided into five zones each 40 µm long and the number of pigmented immunocytes in each zone was counted. Mean and S.E.M. (H) The distribution of Sp-Efn in the same region of ectoderm was determined from a set of mid-sagittal images from 6 embryos prepared with anti-Sp-Efn (See Figure 1—figure supplement 1). Projections of 6-image stacks were prepared and equal-sized rectangles were positioned at 40 µm intervals along the ectoderm. Mean intensity per pixel, normalized to the highest intensity per embryo was determined within each rectangle and Mean and S.E.M. plotted.
-
Figure 1—source data 1
Source data for Figure 1G and H.
- https://doi.org/10.7554/eLife.16000.009
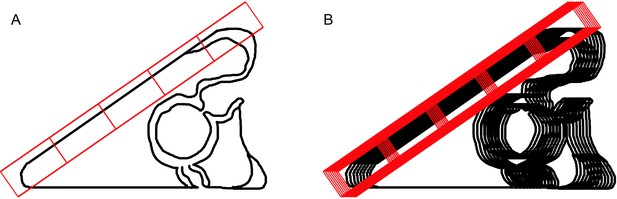
Figure 1G Quantification of pigmented immunocytes.
The distribution of pigmented immunocytes on the dorsal aspect of the ectoderm was determined from a set of mid-sagittal confocal optical sections from 72 hr embryos fixed with methanol and prepared for immunofluorescence with Sp1. Embryos in lateral orientations were selected and a set of 8 optical sections centered on an image plane that included the mouth was collected for each specimen. A maximum intensity projection of the 8 optical sections from 6 specimens was prepared. For each of these 6 projected images, the distance from the posterior-most tip of the larva along the dorsal surface was divided into 5 zones, each 40 µm in length the number of pigment cells in each zone was counted, and the mean and S.E.M. were calculated and plotted. A Sketch showing a mid-sagittal, lateral optical section of a pluteus. (B) Sketch to demonstrate a projection of 8 optical sections centred mid-sagittally. The number of pigment cells within the volume bounded by the rectangular reticle was determined and plotted in Figure 1G. Figure 1H Quantification of Sp-Efn. The distribution of Sp-Efn along the dorsal aspect of the ectoderm was determined from a set of mid-sagittal confocal optical sections from 72 hr embryos fixed with PEM and prepared for immunofluorescence with anti-Sp-Efn (4D2). Embryos in lateral orientations were selected and a set of 8 sections centered on an image plane that included the mouth was collected for each specimen. A median intensity projection of the stack of 8 optical sections from 6 specimens was prepared. Equal-sized reticles were positioned at 40 µm intervals along the dorsal surface. The reticles were positioned so that they included only ectodermal cells and their associated cytonemes. The mean intensity per pixel within each rectangle was determined and the values were normalized to a percentage of the most posterior sample. The mean value for each region and S.E.M. were calculated and plotted.
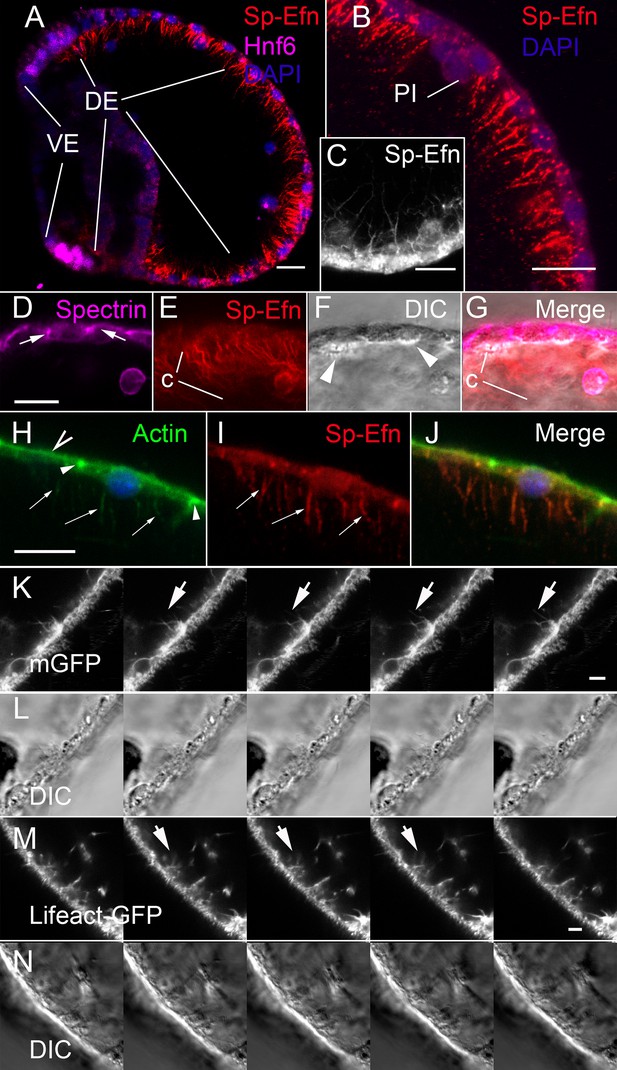
Sp-Efn is expressed on membrane-bounded, actin-containing cytonemes on the blastocoelar surface of dorsal ectoderm.
(A) Fixation that preserves the cytoskeleton (PEM, Vielkind and Swierenga, 1989) reveals that Sp-Efn immunoreactivity localizes to long thin projections from the basal surfaces of dorsal ectoderm (DE). VE, ventral ectoderm Bar = 15 µm. (B,C) Higher magnification showing cellular detail of basal surface of dorsal ectodermal cells. PI, pigmented immunocyte. (B) Z-projection of 5 optical sections of a 72 hr larva. C Single optical section of dorsal ectodermal cells of 48 hr gastrula stage embryo. (D–G) Images demonstrating anti-Sp-Efn immunoreactivity is principally associated with basal cytonemes. (D) Spectrin localizes to apical junctional complexes (arrows). (E) Sp-Efn localizes to thin filamentous projections (c) underlying the dorsal ectoderm. (F) DIC image in which the basal surface (arrowheads) of the ectodermal cells is bright relative to the rest of the cytoplasm, showing that the cytonemes project into the blastocoel. Note that most of the Sp-Efn immunoreactivity is in the cytonemal layer, not in the cytoplasm of the ectodermal cells. Bar = 15 µm (H–J) Sp-Efn localizes to actin containing basal projections of ectoderm. (H) F-actin (Alexa 488-phalloidin) is abundant in the sub-apical cytoplasm of ectoderm (open arrowhead) and junctional complexes (arrowheads). In addition basal projections (arrows) are also fluorescent. (I) Sp-Efn localizes to the same basal projections that bind phalloidin (arrows). Bar = 15 µm (K, L) To determine if there are membrane-bounded cytonemes on the basal surfaces of blastocoelar cells, eggs injected with RNA encoding membrane GFP (mGFP) were live imaged at 4 frames per min for intervals of 45–60 min. Individual thin, fluorescent processes appear to extend from the ectodermal cells (arrows). Bar = 4 µm See supplemental data, Video 6. (M, N) To determine if ectodermal cells extend actin containing cytonemal processes, eggs injected with RNA encoding Lifeact-GFP (Riedl et al., 2008) and live imaged at 4 frames per min for intervals of 45–60 min. In addition to processes emanating from blastocoelar cells, there are thin processes, about 15 µm in length that extend from ectoderm into the blastocoel. Bar = 4 µm See supplemental data, Video 7.
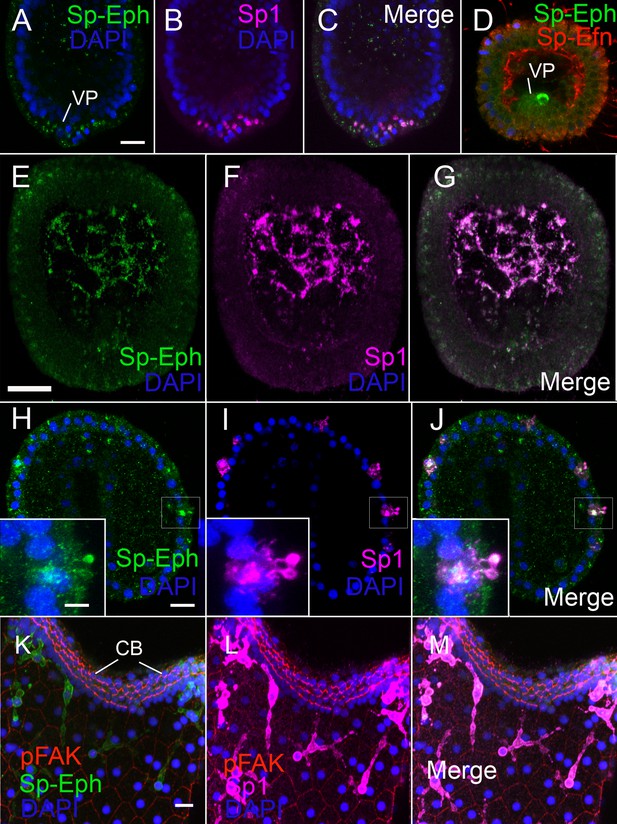
Pigmented immunocytes express Sp-Eph as they differentiate in the vegetal plate and throughout their migration and insertion into ectoderm.
(A–C) In blastulae, SpEph can be detected in cells beginning to express the pigmented immunocyte marker Sp1. The cells are in the vegetal plate and immunoreactivity is strongest in foci adjacent to or overlapping with foci of Sp1 immunoreactivity (MeOH fixation). (D) At stages in which cells expressing Sp-Eph are releasing from the vegetal plate, Sp-Efn can be detected on process on the basal surfaces of ectodermal cells. Here a single pigmented immunocyte progenitor is emerging from the vegetal plate (PEM fixation). (E–G) Pigmented immunocyte precursors expressing surface Sp1 in the blastocoel also express Sp-Eph (MeOH fixation). (H–J) In gastrulae, pigmented immunocytes expressing Sp-Eph have inserted into the ectoderm. Inset images indicate that processes of immunocytes that extend through the ectoderm express Sp-Eph (MeOH fixation). See supplemental data Video 5. (K–M). In early larvae immunocytes within the ectoderm continue to express Sp-Eph on their surface (MeOH fixation). Bars = 15 µm
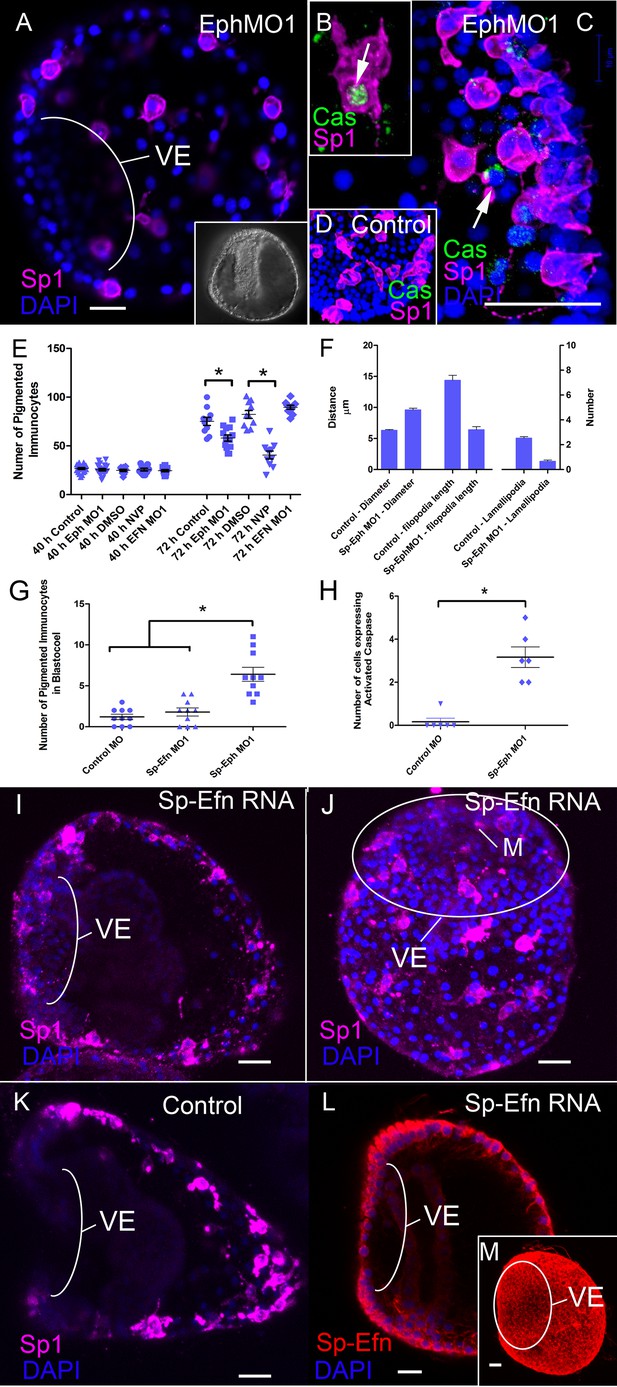
Interfering with expression of Sp-Eph or inhibition of Eph kinase function impedes immunocyte insertion into the ectoderm and they become immunoreactive to anti-Caspase3.
(A) Maximum intensity projection of an embryo injected with Sp-Eph MO1 (MeOH fixation). Pigmented immunocytes are dispersed, with some having inserted into the ectoderm. The immunocytes are more rounded and do not have their typical dendritic form. They are commonly within the blastocoel. The ventral ectoderm (VE) remains clear of immunocytes. Inset: DIC image of another specimen showing the overall healthy appearance of Sp-Eph morpholino injected embryos (MeOH fixation). (B,C) When Sp-EphMO1-injected embryos are prepared with an antibody that recognizes only the cleaved, or activated form of Caspase3, pigmented immunocyte precursors in the blastocoel are immunoreactive (MeOH fixation). (D) Control morpholino injected embryos have almost no pigmented immunocytes that are anti-Caspase3 immunoreactive (MeOH fixation). (E) Counts of the number of Sp1 immunoreactive cells indicate that there are no differences in the number of cells among treatments (NegControlMO, Sp-EphMO1, Sp-EfnMO1, DMSO, or NVP) in prism stages. However, in early plutei there are fewer pigmented immunocytes in Sp-EphMO-injected embryos, or embryos treated with NVP. (F) Interfering with expression of Sp-Eph blocks the transition to epithelial-inserted, dendritic morphology. In Sp-EphMO1 injected embryos, immunocytes have larger diameters, shorter filopodia, and fewer lamellipodia than NegControlMO injected embryos. (G) There are fewer pigmented immunocytes in the blastocoels of 72 hr embryos injected with control MO, or Sp-Efn MO1 than there are in 72 hr embryos injected with Sp-Eph MO1. (H) Preparations of Morpholino injected embryos with an antibody that recognizes the activated form of Caspase3 show that there are significantly more pigmented immunocytes in the blastocoel expressing activated Caspase3. (I–L) Expressing Sp-Efn throughout the embryo results in mislocalization of immunocytes. (I) Lateral view of an embryo injected with 200 ng/µl Sp-Efn RNA. The image is a projection of 8–1 µm optical sections centered on the mouth. Note that Sp1 labelled immunocytes have inserted, or are closely associated with the ventral ectoderm (VE) (MeOH fixation). (J) Maximum intensity projection of the ventral surface of an embryo injected with RNA encoding full length Sp-Efn. Pigmented immunocytes are inserted into the ventral ectoderm (MeOH fixation). See Figure 4—figure supplement 1 for a set of orthogonal projections at various levels through the image stack used to prepare this projection, which demonstrates that the Sp-1 labelled immunocytes are inserted in, or closely associated with the ventral ectoderm. The position of the oval outlining the ventral ectoderm was determined by the higher nuclear density of the cliary band. M; mouth K. Lateral view of an uninjected, control embryo prepared in the same manner as I (MeOH fixation). The image is a projection of 8–1 µm optical sections centered on the mouth. Note that Sp1 labelled immunocytes have not inserted in ventral ectoderm (VE). (L) A single mid-sagittal, optical section of an embryo injected with Sp-Efn RNA, showing immunoreactivity in the ventral ectoderm. Note that at this stage there is almost no expression of Sp-Efn in endoderm and the expression in ventral ectoderm appears graded (PEM fixation). (M) Maximum intensity projection of an embryo injected with Sp-Efn RNA showing ectopic expression of Sp-Efn in the ventral ectoderm (PEM fixation). * indicates significantly different outcomes Bars = 10 µm.
-
Figure 4—source data 1
Source data for Figure 4E–H.
- https://doi.org/10.7554/eLife.16000.016
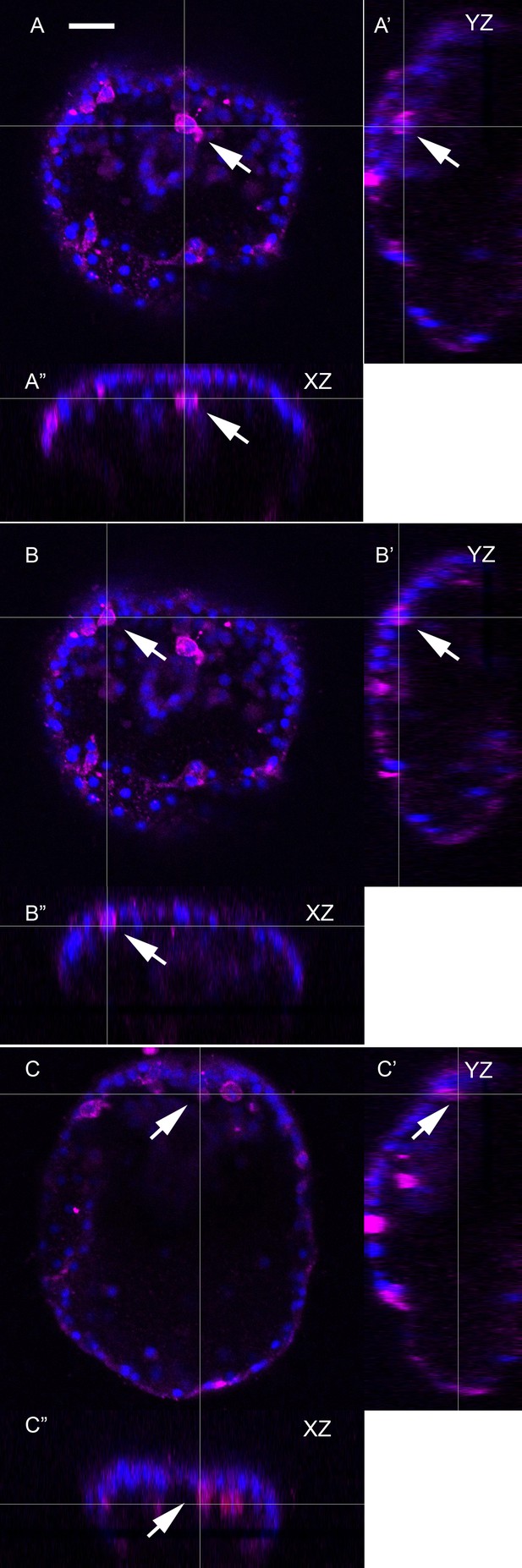
Orthogonal views of Figure 4J, a maximum intensity projection of an embryo expressing Sp-Efn throughout the ectoderm.
This supplemental figure is comprised of 3 sets of orthogonal views (YZ and XZ) of the image stack used to make the Figure 4J projection. (A–A”) The thin lines indicate the image plane of the orthogonal view and demonstrates that the pigmented immunocyte positioned immediately below the mouth in Figure 4J is associated with the ventral ectoderm (arrow). Similarly in (B–B”) and (C–C”) all of the immunocytes that appear to be in ventral ectoderm in the projected image (Figure 4J) are within or contacting the ventral ectoderm and do not lie deeper in the embryo. Bar = 15 µm.
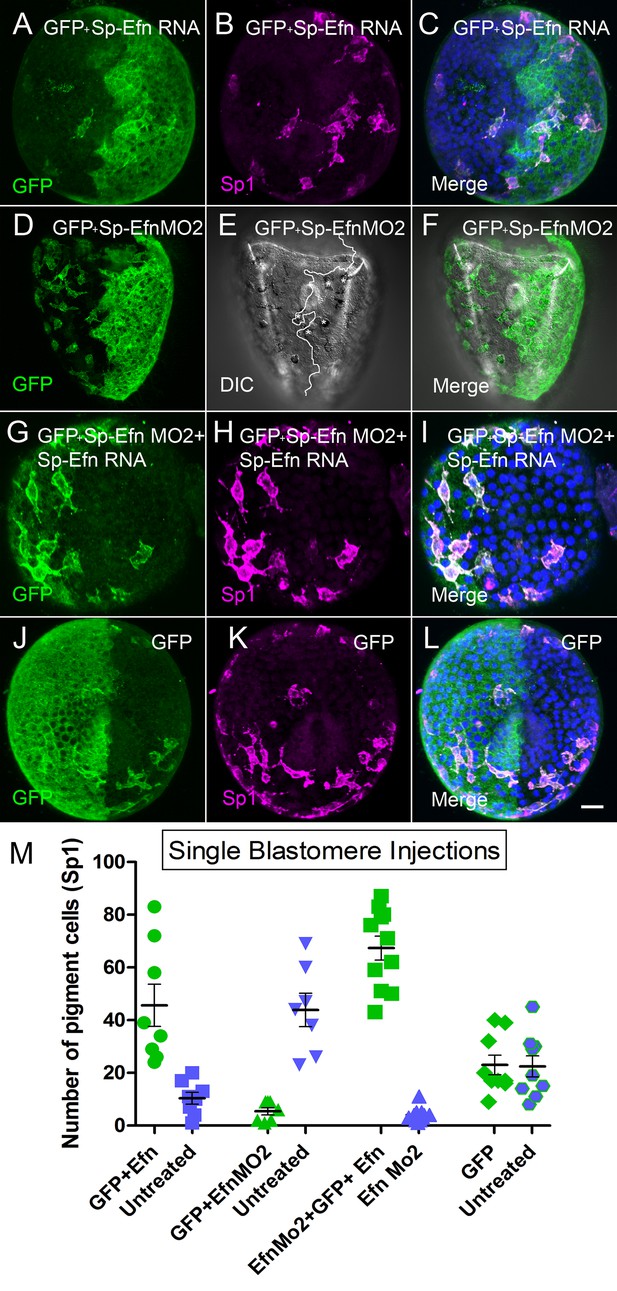
Altering the abundance of Sp-Efn in half embryos by injecting a single blastomere of 2-cell embryos indicates pigmented immunocytes insert preferentially in ectoderm expressing Sp-Efn and high levels of expression of Sp-Efn enhances pigment cell insertion.
(A–C) Maximum intensity projection of an embryo in which half of the specimen co-expresses membrane GFP and Sp-Efn (MeOH fixation). Sp1 reveals the distribution of immunocytes. Note that a subset of the immunocytes expresses GFP. Most of the immunocytes are either inserted in the half expressing Sp-Efn or extend contacts to that half of the embryo. (D–F) Through focus projection of a living embryo that was co-injected in one blastomere with GFP and SpEfn MO2. In the DIC image immunocytes can be identified by their pigment and the white line demarks the interface between ectoderm containing morpholino (anatomical left) and untreated ectoderm (anatomical right). A subset of the immunocytes express GFP. Several pigmented immunocytes are associated with the interface between the to domains of ectoderm, those marked with * project processes to the untreated, Sp-Efn expressing, ectoderm. (G–I) Embryo from an egg that was injected with Sp-EfnMO2 to suppress Sp-Efn expression throughout the embryo (MeOH fixation). Once the egg had cleaved, one blastomere was injected with Sp-Efn RNA. The pigmented immunocytes are almost exclusively inserted in the half of the embryo expressing Sp-Efn. (J–L) Control embryos were injected with mGFP RNA only and the distribution of pigmented immunocytes can be seen to be unaffected (MeOH fixation). (M) Quantification of the distribution of pigmented immunocytes from the experiments depicted above (A–L). For each treatment (X axis) there is an injected half (green) and an uninjected half (blue). The number of pigmented immunocytes inserted in ectoderm of the injected half, or the uninjected half was determined for each embryo (72 hr). Bar = 10 µm
-
Figure 5—source data 1
Source data for Figure 5M.
- https://doi.org/10.7554/eLife.16000.019
Videos
Epithelial-mesenchyme transition (EMT) and migration of DGP:GFP labelled pigmented immunocytes.
Live imaging of an embryo (40 hr) injected with DGP:GFP (Ransick and Davidson, 2012). GFP is expressed in pigmented immunocyte precursors undergoing EMT (first arrow). Subsequent to this cell migrating to the ectoderm, another cell (second arrow) undergoes EMT and also attaches to the ectoderm. The duration of the sequence is 90 min.
Pigmented immunocytes remain at the site of insertion for prolonged periods of time.
Live imaging of the pigmented immunocytes in an early pluteus. The cells remain active, but they are not displaced from their location throughout the 1.5 hr sequence. As pigmented immunocytes remain relatively stationary, we concluded that they select a site to insert during the phase of migration subsequent to release from the vegetal plate.
Pigmented immunocyte inserting into ectoderm.
In the insertion sequence a cell first approaches the ectoderm, then inserts between epithelial cells (30 min sequence).
Pigmented immunocyte inserts into ectoderm and changes from migratory form to a form similar to a dendritic cell.
The sequence is 90 min long, beginning with a 48 hr embryo.
In this sequence, a pigmented immunocyte that is inserted in the ectoderm extends a single process through the ectoderm to the outside of the embryo (arrow).
The process extends and retracts repeatedly throughout this 30 min sequence. Note also the distinctive process extended from the blastocoelar surface of the immunocyte.
Membrane GFP live imaging of dorsal ectodermal cytonemes.
Live imaging of membrane GFP expressing ectodermal cells. There are numerous thin, membrane-bounded processes extending and retracting from the basal surface of dorsal ectodermal cells. (30 min sequence).
Lifeact-GFP live imaging of dorsal ectodermal cytonemes.
Live imaging of ectodermal cells in an embryo expressing Lifeact: GFP. There are numerous thin, actin containing processes extending and retracting from the basal surface of dorsal ectodermal cells. (30 min sequence).
Behavior of pigmented immunocyte in the context of mosaic Sp-Efn expression.
In this 90 min sequence half of the embryo is expressing high levels of Sp-Efn, as indicated by the regions co-expressing GFP. Pigmented immunocytes have inserted in the ectoderm adjacent to the interface. Once inserted they do not move toward the region expressing higher levels of Sp-Efn. This sequence supports a model in which pigmented immunocytes migrate and then insert in the ectoderm without making extensive subsequent movements within the ectoderm.





