First bone-cracking dog coprolites provide new insight into bone consumption in Borophagus and their unique ecological niche
Figures
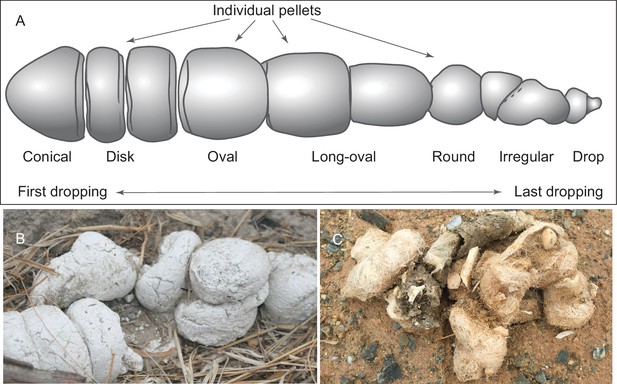
(A) morphology of individual pellets in a complete assemblage of feces from a single dropping event by the extant spotted hyena, Crocuta crocuta. Adapted from ([Diedrich, 2012]:Figure 4), except for the orientation (Diedrich’s anterior/posterior orientation is counter to traditional sense of anatomy). (B) scats of extant spotted hyena (still image reproduced from a Smithsonian magazine video, available at http://www.smithsonianmag.com/videos/category/weird-science/weird-science-hyena-poop/?no128ist). (C) scats of extant grey wolf; note preservation of bone fragments and hairs (photo by Xiaoming Wang on September 21, 2016 in Xorkol Basin in southern Xinjiang Uygur Autonomous Region, China).
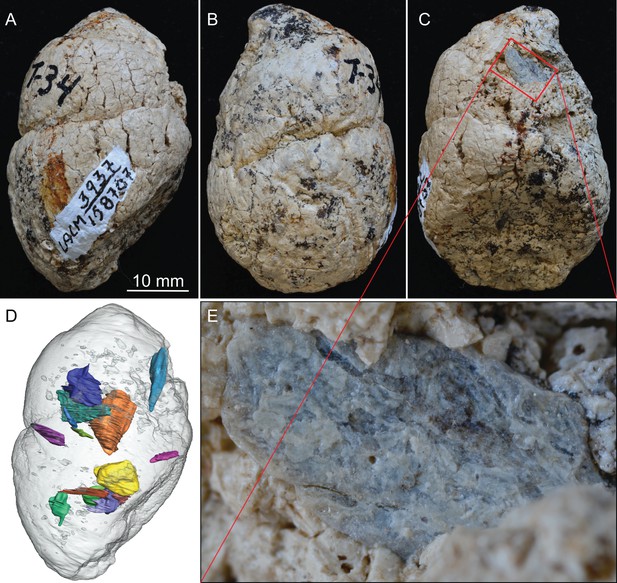
LACM 158707, a complete coprolite from LACM locality 3937 (=Turlock Lake 34), Mehrten Formation, Stanislaus County, California, collected by Dennis Garber.
(A) Lateral view, top is toward distal (first dropping) end; (B) another lateral view about 90° from A; (C) another lateral view about 90° of further rotation from B; (D) 14 bone fragments (in various colors) digitally segmented within the coprolite (light grey) in the same orientation as in A; (E) close-up of an exposed bone fragment (unidentified) on C showing acid etching (flaking) on external surface. See also Video 1 to show three dimensional relationships of individual bones within this coprolite.
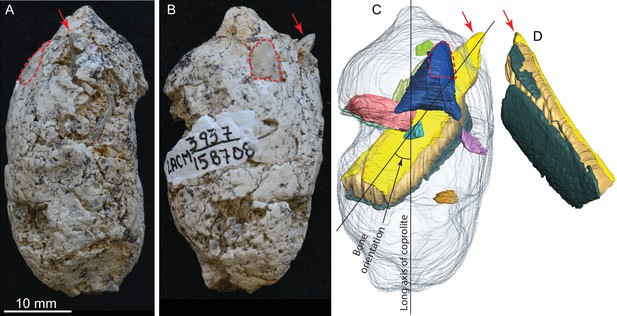
LACM 158708, a complete coprolite and bones contained within, from LACM locality 3937, Mehrten Formation, Stanislaus County, California, collected by Dennis Garber.
(A) Lateral view, top is toward distal (first dropping) end; (B) another lateral view about 90° from A; (C) digitally separated individual bones (in different colors) within coprolite matrix (light grey), identical view as that of B; (D) a rotated view of a rib fragment seen in C, showing the convex (external) side, yellow and dark green shapes representing internal (toward chest cavity) and external cortical bone respectively, and yellowish brown sandwiched between the cortical bones being cancellous bone. Red arrows indicate the same protruded tip of rib fragment, and red dashed lines define the exposed outlines of a flat bone (mostly buried within coprolite matrix; dark blue piece in C shows the full extent of this bone within the coprolite). With the exception of the rib, all other bone fragments are unidentifiable. See also Video 2 and original Avizo segmentation file (web link) to show three dimensional relationships of individual bones within this coprolite.
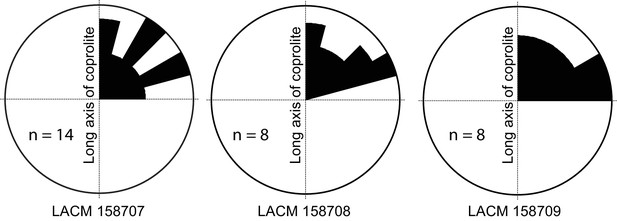
Rose diagram of bone orientations inside the coprolites.
Only coprolite pellets with at least eight bone fragments inside and a clear long axis are presented. (In the case of LACM 158709, see Figure 3A: although its axial dimension is similar to its diameter, its constricted distal end gives unambiguous orientation of its long axis.) Angles (0–90°) are between the long axis of the coprolite and the long axis of bone fragments in three-dimensional space (see Figure 3C for a definition of the angles). Data from Table 1.
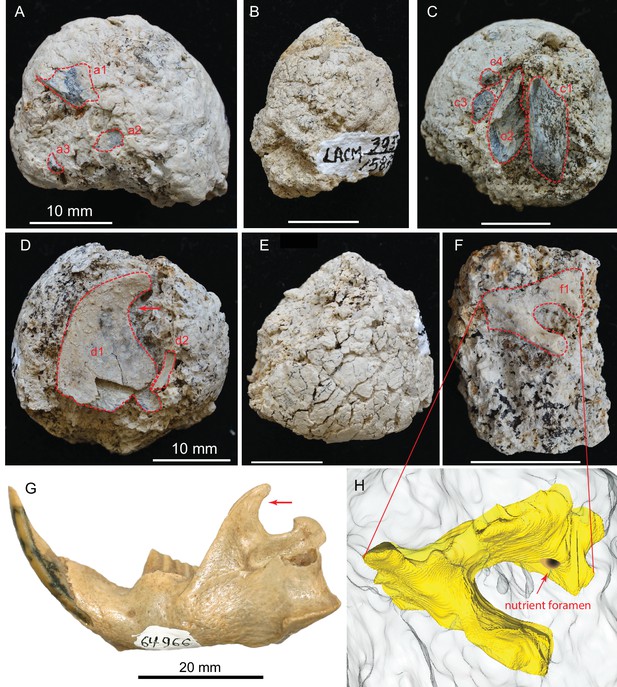
(A) LACM 158709 with three visible bone fragments (a1, a2, a3); (B) LACM 158710; (C) LACM 158711 with four visible bone fragments partially prepared (c1–c4); (D) LACM 158712 with two visible bone fragments partially exposed (d1, d2); (E) LACM 158713, surface cracks suggesting desiccation before burial; (F) LACM 158716 with one bone fragment partially exposed (f1); (G) left jaw of extant Eucastor tortus, compared to the fragment of coronoid process of the mandible (red arrows) of d1 in D (FMNH 64966; photo courtesy of Joshua Samuels); (H) digitally reconstructed bone (colored yellow; light grey background is coprolite matrix) of f1 in F, tentatively identified as the ventral aspect of the foramen ovale in the basisphenoid of a medium-sized mammal. Dashed red lines indicate exposed outlines of bones. All scales for coprolites are 10 mm.
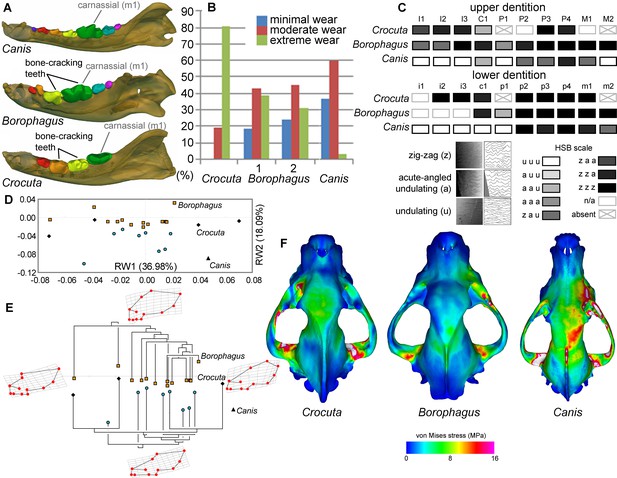
Comparison of craniodental functional morphology in Canis, Borophagus, and Crocuta.
(A) Lower dentition homology and positions of functionally analogous bone-cracking teeth. Jaws are scaled to the same length. (B) Macrowear data from lower first molar samples of Crocuta crocuta (Sub-Saharan Africa) (data from [DeSantis et al., 2017]), Borophagus parvus (new data based on AMNH specimens from (1) Quibiris Formation, Arizona and (2) Big Sandy Formation, Arizona), and Canis lupus (new data based on AMNH specimens from Alberta, Canada). (C) Hunter-Schreger Band (HSB) enamel microstructure patterns in the upper and lower dentitions of the three carnivorans; darker shades indicate higher degree of zig-zag HSB specialization (modified from Figure 2 from [Tseng, 2011]). (D) Morphospace of relative warp (RW) axes from a geometric morphometric analysis of fossil (shaded symbols) and extant (black symbols) canid and hyaenid cranial shape, and (E) Phylogenetic relationships of borophagine canids (top) and hyaenids (bottom) plotted onto morphometric data, with Canis indicated by black triangle. Both (D) and (E) are modified from Figure 5 from Tseng and Wang (2011). (F) von Mises stress distributions in the crania during right fourth premolar bite simulations using 3-D finite element analysis, with warmer colors indicating higher stress. Crania are scaled to the same length (modified from Figure 7 from [Tseng, 2013]).
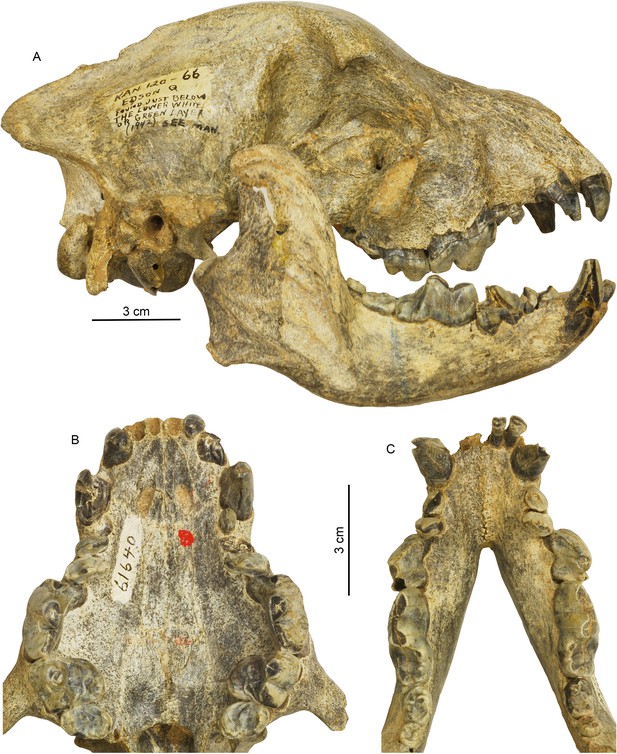
Cranial and dental morphology of Borophagus secundus (F:AM 61640 from Edson Quarry, Marshall Ranch, Sherman County, Kansas, late Hemphillian).
A suite of features is commonly associated with bone-crushing adaptations, such as a highly vaulted forehead, shortened rostrum and associated imbrication of premolars, thickened lower jaws, broadened palate, laterally flared lower cheek teeth, differentially enlarged P4 relative to P3 and p4 relative to p3, and anterior premolars (P1-3 and p1-3) reduced to small pegs that are no longer functioning in occlusion. (A) right lateral view of skull and mandible; (B) occlusal view of upper teeth; and (C) occlusal view of lower teeth.
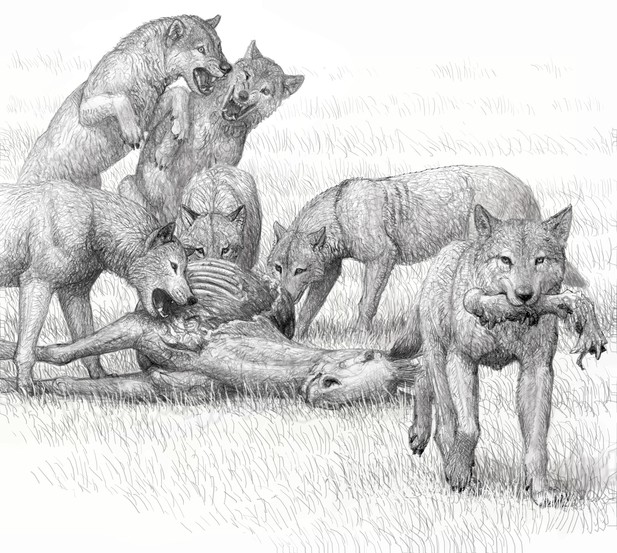
Artist conception of feeding by a pack of bone-crushing dogs of the species Borophagus secundus, sister taxon of Borophagus parvus, by Mauricio Antón.
Competitive group feeding does not permit leisurely picking and choosing of meat for quiet consumption and may have been a driving force for complete utilization of carcasses. Adapted from Wang et al., 2008: figure 5.4 and with permission for reproduction by Mauricio Antón.
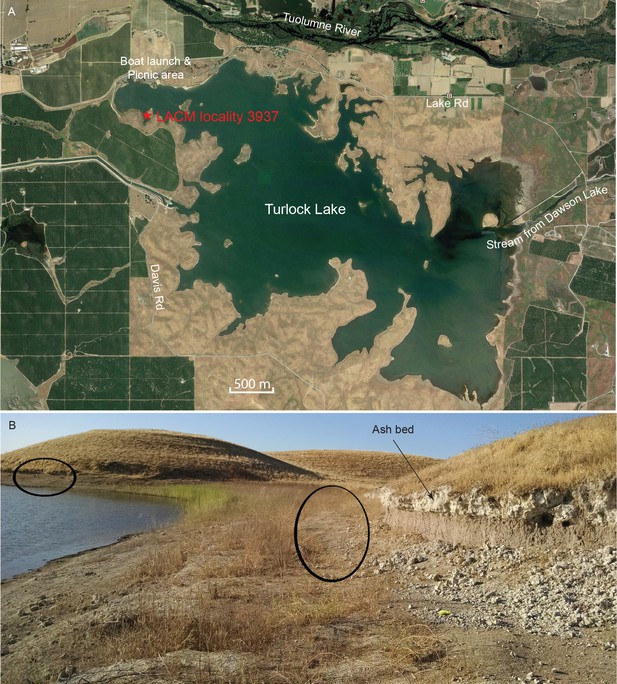
Map and photo of coprolite locality.
(A) satellite image of Turlock Lake area (37°36–37'N 120°34–36'W) from Google Earth Pro, image date March 31, 2015 (Google Earth Pro (Version 7.1.5.1557), [Google Inc, 2015]); red star is approximate position of LACM locality 3937 (=Dennis Garber T-34 locality) and of LACM locality 3935 (=Dennis Garber T-32 locality). (B) LACM locality 3937, looking to the south; black ovals are approximate positions of fossil-producing horizons and that to the left is the location for coprolites; photograph by Jacob Biewer on September 5, 2015.
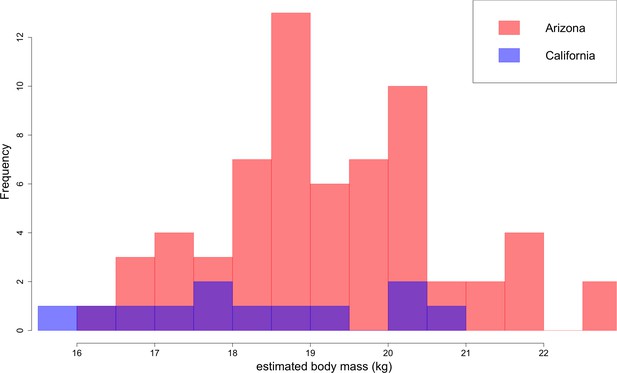
Distribution of B. parvus body mass estimated from lengths of the lower first molar (carnassial) using the equation from Van Valkenburgh (1990).
The Arizona population tends to be larger in body size than the California population, which largely comprises Turlock Lake individuals.
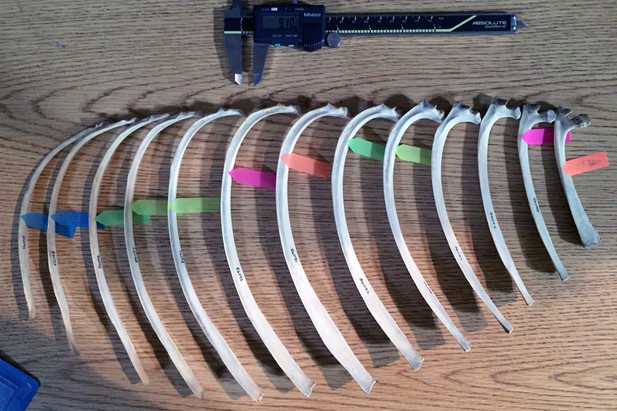
Rib measurement methods illustrated on half of a ribcage of Eld’s deer (Cervus eldi).
Anterior ribs are to the right; posterior, to the left. For this set of measurements, the colored tags mark where each rib measures 9.1 mm in anteroposterior width. The corresponding mediolateral thickness at the marked points were then recorded. Ribs without a colored tag were either wider or narrower for much of its length than the two fixed measurements of coprolite width and thickness.
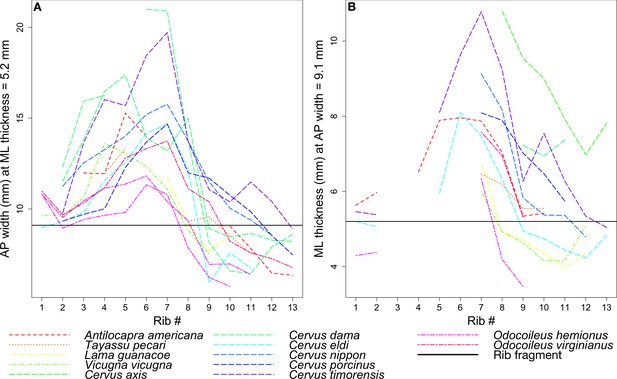
Rib measurements per species.
The horizontal black line in both plots indicates the corresponding measurement for the coprolite rib fragment. (A) Anteroposterior width of the rib where it has a mediolateral thickness of 5.2 mm, the rib fragment thickness. (B) Mediolateral thickness of the rib where it has an anteroposterior width of 9.1 mm, the rib fragment width.
Videos
LACM 158707 movie: A video of microCT scan of LACM 158707 with variously colored bones digitally segmented within the coprolite.
Video in Avizo Lite 9.2 by Stuart C. White.
LACM 158708 movie: A video of microCT scan of LACM 158708 with variously colored bones digitally segmented within the coprolite.
Video in Avizo Lite 9.2 by Stuart C. White.
Tables
Measurements of coprolites and their included bones.
Maximum diameter and length of coprolites are measured by digital calipers, and the rest are calculated by Avizo software. ‘*' in coprolite diameter and length indicates incomplete dimensions due to damage.
| Coprolite dimensions | Bone dimensions | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LACM catalogue number | Maximum diameter × length (mm) | Coprolite volume (mm3) | Matrix volume (mm3) | Matrix fraction | Bone fragments contained | Bone max length (mm) | Bone max width (mm) | Bone orientation (degrees) | Bone volume (mm3) | Bone fraction/coprolite | ||||
| 158706 | 24.6* × 31.9* | 5871 | 5871 | 100% | None | |||||||||
| 158707 | 31.2 × 47.2 | 18508 | 17823 | 96% | Bone_1 | 8.1 | 6.6 | 70 | 155 | |||||
| Bone_2 | 16.5 | 5.1 | 24 | 56 | ||||||||||
| Bone_3 | 10.6 | 7.8 | 8 | 207 | ||||||||||
| Bone_4 | 6.1 | 5.1 | 70 | 23 | ||||||||||
| Bone_5 | 4.6 | 4.6 | 63 | 26 | ||||||||||
| Bone_6 | 7.9 | 7.2 | 10 | 43 | ||||||||||
| Bone_7 | 3.6 | 2.4 | 32 | 11 | ||||||||||
| Bone_8 | 11.4 | 4.7 | 12 | 45 | ||||||||||
| Bone_9 | 5.5 | 1.7 | 85 | 3 | ||||||||||
| Bone_10 | 4.7 | 2.8 | 40 | 5 | ||||||||||
| Bone_11 | 11.0 | 2.2 | 68 | 8 | ||||||||||
| Bone_12 | 8.5 | 4.8 | 43 | 78 | ||||||||||
| Bone_13 | 5.0 | 3.9 | 58 | 10 | ||||||||||
| Bone_14 | 6.1 | 3.6 | 37 | 14 | ||||||||||
| Total bone | 685 | 4% | ||||||||||||
| 158708 | 24.9 × 44.6 | 10184 | 8814 | 87% | Bone_1 | 3.0 | 2.1 | 21 | 3 | |||||
| Bone_2 | 11.1 | 4.4 | 15 | 25 | ||||||||||
| Bone_3 | 16.1 | 10.0 | 14 | 344 | ||||||||||
| Bone_4 | 5.6 | 3.4 | 39 | 11 | ||||||||||
| Bone_5 Cortex | 8.3 | 7.7 | 61 | 14 | ||||||||||
| Bone_5 Marrow | 9.7 | 6.8 | 61 | 104 | ||||||||||
| Bone_6 | 5.1 | 3.4 | 49 | 11 | ||||||||||
| Bone_7 | 4.3 | 2.1 | 70 | 6 | ||||||||||
| Bone_8 Rib long | 31.0 | 7.0 | 46 | 156 | ||||||||||
| Bone_8 Rib Marrow | 30.1 | 8.2 | 46 | 574 | ||||||||||
| Bone_8 Rib short | 29.4 | 8.7 | 46 | 122 | ||||||||||
| Total bone | 1370 | 13% | ||||||||||||
| 158709 | 27.1 × 23.4 | 6556 | 6341 | 97% | Bone_1 | 12.5 | 2.3 | 65 | 21 | |||||
| Bone_2 | 7.9 | 4.5 | 80 | 45 | ||||||||||
| Bone_3 | 12.6 | 4.4 | 24 | 53 | ||||||||||
| Bone_4 | 6.6 | 2.3 | 45 | 10 | ||||||||||
| Bone_5 | 5.0 | 2.2 | 54 | 10 | ||||||||||
| Bone_6 | 3.6 | 1.9 | 72 | 5 | ||||||||||
| Bone_7 | 6.1 | 4.5 | 11 | 28 | ||||||||||
| Bone_8 | 10.6 | 4.9 | 81 | 42 | ||||||||||
| Total bone | 214 | 3% | ||||||||||||
| 158710 | 21.3 × 26.6 | 4066 | 4066 | 100% | None | |||||||||
| 158711 | 29.1* × 31.2* | 11741 | 11251 | 96% | Bone_1 | 16.9 | 7.1 | 197 | ||||||
| Bone_2 | 18.7 | 6.3 | 93 | |||||||||||
| Bone_3 | 6.8 | 3.0 | 13 | |||||||||||
| Bone_4 | 13.1 | 7.1 | 169 | |||||||||||
| Bone_5 | 6.5 | 3.7 | 18 | |||||||||||
| Total bone | 490 | 4% | ||||||||||||
| 158712 | 29.4 × 27.5* | 8284 | 8012 | 97% | Bone_1 | 21.3 | 12.3 | 37 | 234 | |||||
| Bone_2 | 13.2 | 3.1 | 67 | 18 | ||||||||||
| Bone_3 | 7.1 | 2.5 | 2 | 7 | ||||||||||
| Bone_4 | 3.4 | 1.9 | 18 | 2 | ||||||||||
| Bone_5 | 8.6 | 2.8 | 75 | 12 | ||||||||||
| Total | 272 | 3% | ||||||||||||
| 158713 | 27.5 × 25.6* | 8694 | 8454 | 97% | Bone_1 | 15.2 | 6.5 | 26 | 107 | |||||
| Bone_2 | 10.4 | 8.7 | 44 | 114 | ||||||||||
| Bone_3 | 4.7 | 1.9 | 66 | 5 | ||||||||||
| Bone_4 | 4.9 | 2.7 | 72 | 14 | ||||||||||
| Total bone | 240 | 3% | ||||||||||||
| 158714 | 17.7* × 20.9* | 1570 | 1508 | 96% | Bone_1 | 8.2 | 5.2 | 29 | 62 | 4% | ||||
| 158715 | 18.0* × 24.0* | 2481 | 2443 | 98% | Bone_1 | 71 | 39 | 2% | ||||||
| 158716 | 20.5* × 14.9* | 1245 | 1197 | 96% | Bone_1 | 10.2 | 9.0 | 70 | 48 | 4% | ||||
| 158717 | 18.7* × 19.6* | 1424 | 1071 | 75% | Bone 1 | 18.9 | 12.7 | 14 | 353 | 25% | ||||
| Total | 76851 | Total | 3773 | 5% | ||||||||||
Postcranial specimens used to approximate prey body size based on dimensions of the coprolite rib fragment.
https://doi.org/10.7554/eLife.34773.011| Family | Genus | Species | Specimen number |
|---|---|---|---|
| Antilocapridae | Antilocapra | americana | LACM 30482 |
| Tayassuidae | Tayassu | pecari | LACM 86904 |
| Camelidae | Lama | guanacoe | LACM 31328 |
| Camelidae | Vicugna | vicugna | LACM 54706 |
| Cervidae | Cervus | axis | LACM 529 |
| Cervidae | Cervus | dama | LACM 30452 |
| Cervidae | Cervus | dama | LACM 30876 |
| Cervidae | Cervus | eldi | LACM 86095 |
| Cervidae | Cervus | nippon | LACM 31069 |
| Cervidae | Cervus | porcinus | LACM 85966 |
| Cervidae | Cervus | timorensis | LACM 86012 |
| Cervidae | Odocoileus | hemionus | LACM 307 |
| Cervidae | Odocoileus | hemionus | LACM 30903 |
| Cervidae | Odocoileus | virginianus | LACM 52442 |
Additional files
-
Supplementary file 1
158708 bin=4 Avizo file (plus data folder): Segmentation file for LACM 158708 in Avizo software (ThermoFisher Scientific).
Voxel size has been downgraded to 108 micrometers on a side to reduce file size. Segmentation in Avizo Lite 9.2 by Stuart C. White.
- https://doi.org/10.7554/eLife.34773.019
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34773.020





