Plant secondary metabolite-dependent plant-soil feedbacks can improve crop yield in the field
Figures
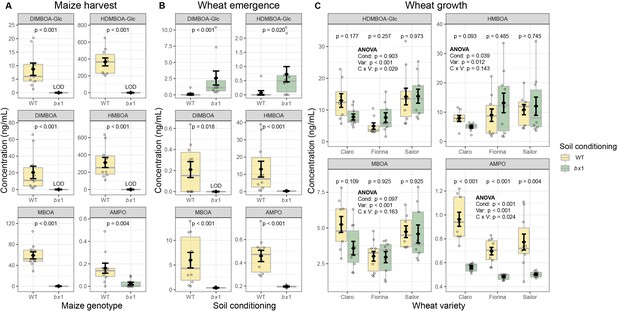
Benzoxazinoid soil conditioning results in persistent chemical fingerprints.
(A) Concentrations of benzoxazinoids in field soil at harvest of wild type (WT) or benzoxazinoid-deficient bx1 mutant maize plants. (B) Benzoxazinoids in field soil 6 weeks after maize harvest. For (A) and (B) means ±SE, boxplots, and individual datapoints are shown and Wilcoxon rank-sum tests are included (FDR-corrected p values, n=10). (C) Benzoxazinoids in field soil at wheat growth. Means ±SE, boxplots, and individual datapoints are shown (n=10). ANOVA tables and pairwise comparisons within each wheat variety are included (FDR-corrected p values). LOD: below limit of detection. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Note that in (C) the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
-
Figure 1—source data 1
Data of benzoxazinoid concentrations shown in Figure 1 and of maize shoot dry weight and soil nutrient levels at maize harvest shown in Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/84988/elife-84988-fig1-data1-v1.xlsx
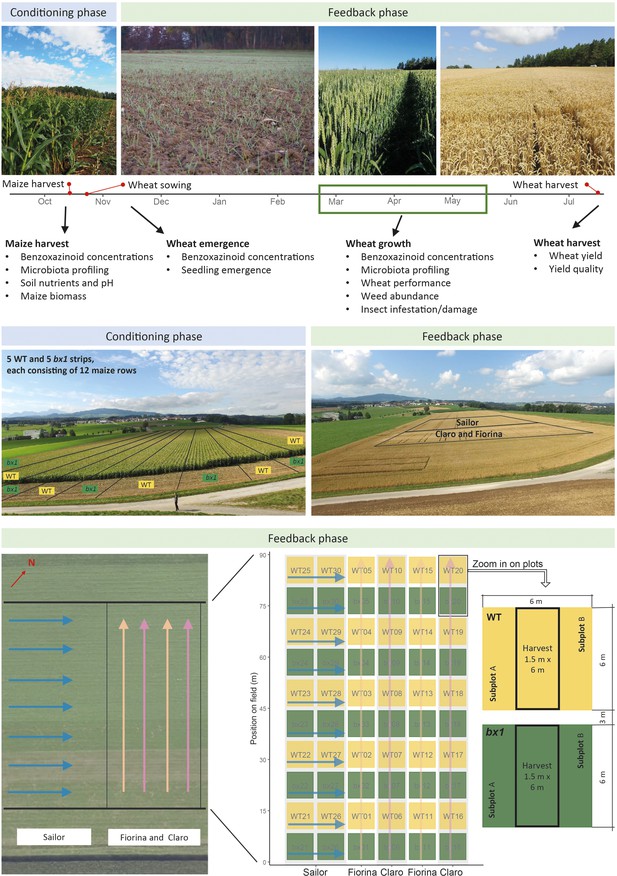
Experimental set-up.
A two-year field experiment was conducted in Posieux, Switzerland. In the first season (conditioning phase) the field was cultivated with 10 strips of maize where wild type (WT, n=5) and the benzoxazinoid-deficient bx1 mutant (n=5) maize were sown alternatingly. Each strip consisted of 12 rows of maize plants. Soil conditioning refers to the process of root benzoxazinoid exudation and the resulting changes in the soil. The impact of soil conditioning was evaluated by analysis of microbiomes, soil benzoxazinoid concentrations, soil nutrients, and pH. In the second season (feedback phase), after the maize was harvested, three wheat varieties (Claro, Fiorina, and Sailor) were sown. Most wheat phenotypes were measured in all subplots, indicated in the zoomed plots. Feedbacks of benzoxazinoid soil conditioning on wheat seedling emergence, growth, and defense were surveyed. Microbial and chemical (benzoxazinoid) soil legacies were again analyzed during wheat growth. At wheat harvest, wheat yield and yield quality were determined. For more details see the method description.
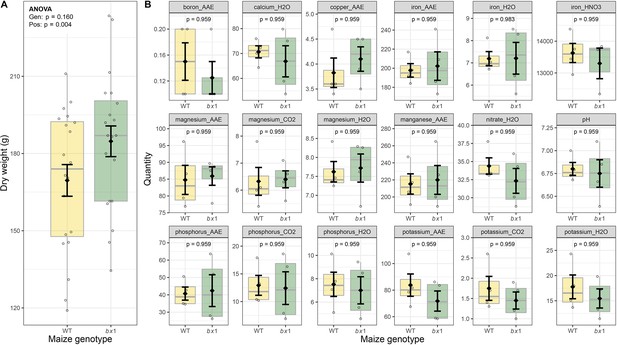
Additional parameters maize harvest.
(A) Shoot dry weight of individual wild type (WT) and bx1 maize plants at harvest. Means ±SE, boxplots, and individual datapoints are shown (n=20). ANOVA table is included. (B) Soil nutrient levels at the end of the maize conditioning phase. Except for pH all values are concentrations in mg/kg soil. Means ±SE, boxplots, and individual datapoints are shown (n=4). Welch’s two-sample t-tests are included (FDR-corrected p values). Gen: maize genotype (WT or bx1). Pos: position on the field. AAE: ammonium acetate EDTA extraction; H2O: water extraction; CO2: carbon dioxide saturated H2O extraction. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
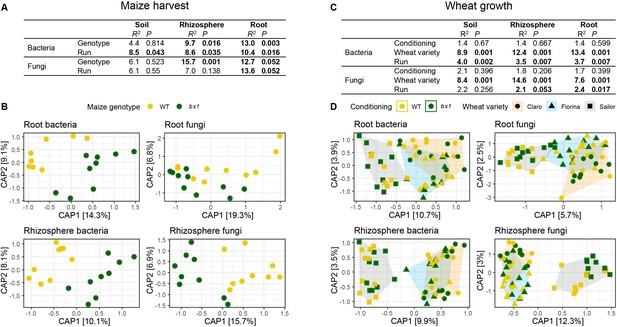
Benzoxazinoid soil conditioning transiently structures rhizosphere microbial communities.
Soil, rhizosphere, and root-associated microbial communities at maize harvest (A, B) and during wheat growth (C, D). (A) Output of PERMANOVA on Bray-Curtis dissimilarities of bacteria and fungi showing R2 and p values for genotype and sequencing run effects in soil, rhizosphere, and root compartments. Significant effects are indicated in bold. (B) Constrained Analysis of Principal Coordinates (CAP) confirming the genotype effects found in the PERMANOVA, axis labels denote percentage of explained variance (n=8–10). (C, D) Same as in (A, B) but also including the factor wheat variety (n=6–10).
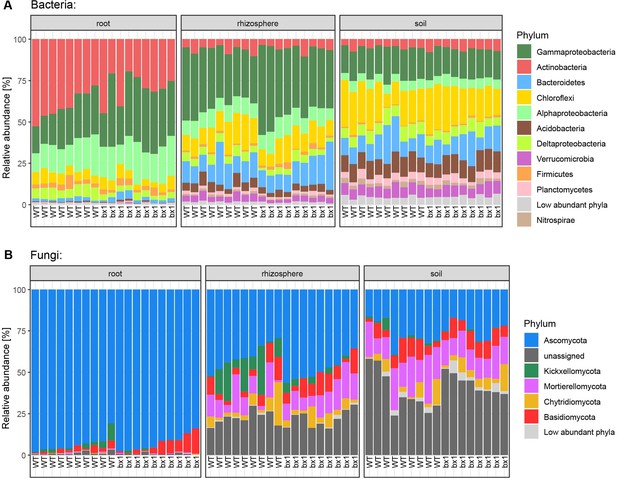
Relative abundance of microbial phyla at maize harvest.
(A) Taxonomy of bacteria and (B) fungi in roots, rhizospheres, and soil of wild type (WT) or benzoxazinoid-deficient bx1 mutant maize plants. All samples are shown.
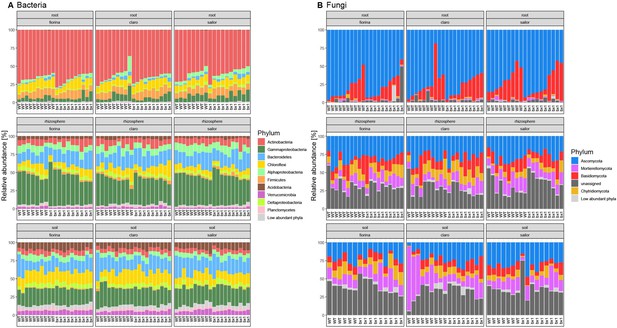
Relative abundance of microbial phyla in the wheat feedback phase.
(A) Taxonomy of bacteria and (B) fungi in roots, rhizospheres, and soils in three wheat varieties grown on wild type (WT) or benzoxazinoid-deficient bx1 mutant conditioned soil. All samples are shown.
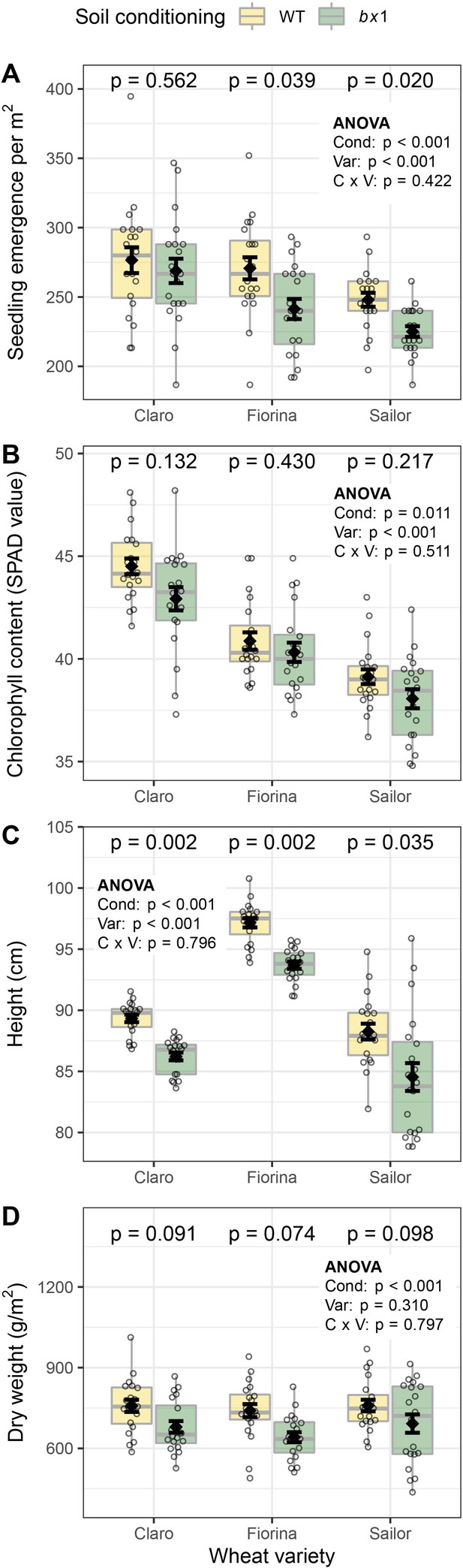
Benzoxazinoid soil conditioning improves wheat emergence and growth.
(A) Seedling emergence, (B) chlorophyll content, (C) plant height, and (D) shoot dry weight of three wheat varieties sown in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize. Means ±SE, boxplots, and individual datapoints are shown (n=20). ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
-
Figure 3—source data 1
Data of phenotypes shown in Figure 3 and Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/84988/elife-84988-fig3-data1-v1.xlsx
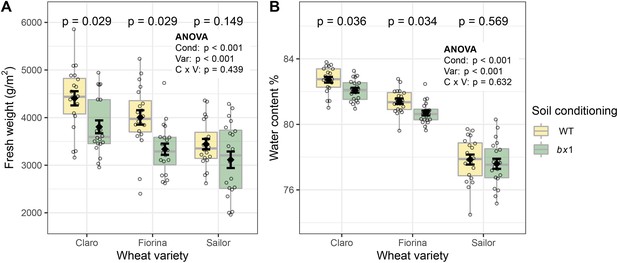
Additional benzoxazinoid soil conditioning effects on wheat growth.
(A) Shoot fresh weight of three wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize. (B) Shoot water content. Means ±SE, boxplots, and individual datapoints are shown (n=20). ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
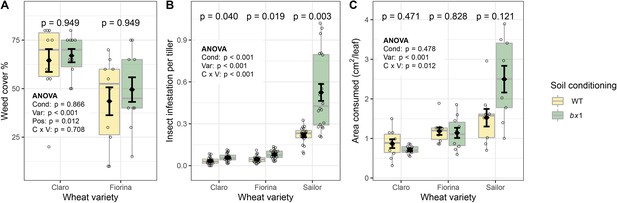
Benzoxazinoid soil conditioning does not change weed pressure, but reduces insect infestation.
(A) Total ground cover by weed plants in plots of three wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize (n=10). No weeds were detected in plots with the variety Sailor due to herbicide treatment of this variety. (B) Mean abundance of cereal leaf beetles (Oulema melanopus) per tillers (n=20) and (C) Consumed flag leaf area by cereal leaf beetles (n=9–10). Means ±SE, boxplots and individual datapoints (n=20) are shown. ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Pos: position on the field. Note that in (A) the minimum value of the y-axis was set to a value greater than zero for clearer visualization of treatment differences.
-
Figure 4—source data 1
Data of phenotypes shown in Figure 4 and of leaf phytohormone levels shown in Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/84988/elife-84988-fig4-data1-v1.xlsx
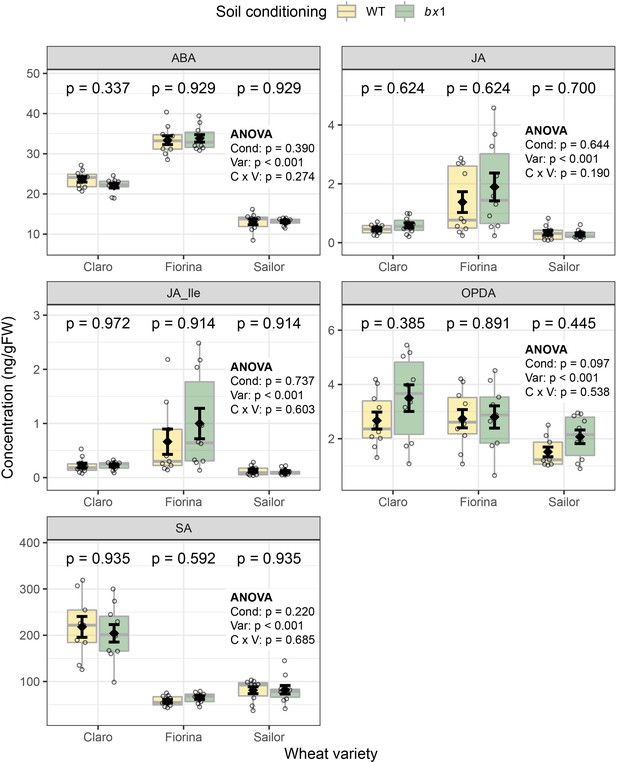
Benzoxazinoid soil conditioning does not affect leaf phytohormone levels of wheat.
Phytohormone levels of three wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize were measured (n=9–10). Means ±SE, boxplots, and individual datapoints are shown. ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. FW: fresh weight. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Pos: position on the field. ABA: abscicic acid. JA: jasmonic acid. JA-Ile: jasmonic acid-isoleucine. OPDA: oxophytodienoic acid. SA: salicylic acid. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
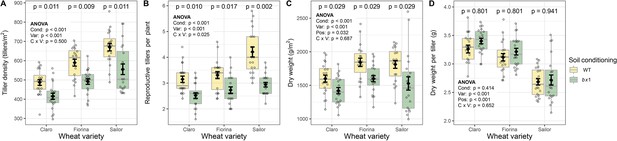
Benzoxazinoid soil conditioning increases wheat density and biomass.
(A) Tiller density, (B) reproductive tillers per plant, (C) shoot dry weight, and (D) dry weight per tiller of the three wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize. Means ±SE, boxplots, and individual datapoints (n=20) are shown. ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Pos: position on the field. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
-
Figure 5—source data 1
Data of phenotypes shown in Figure 5.
- https://cdn.elifesciences.org/articles/84988/elife-84988-fig5-data1-v1.xlsx
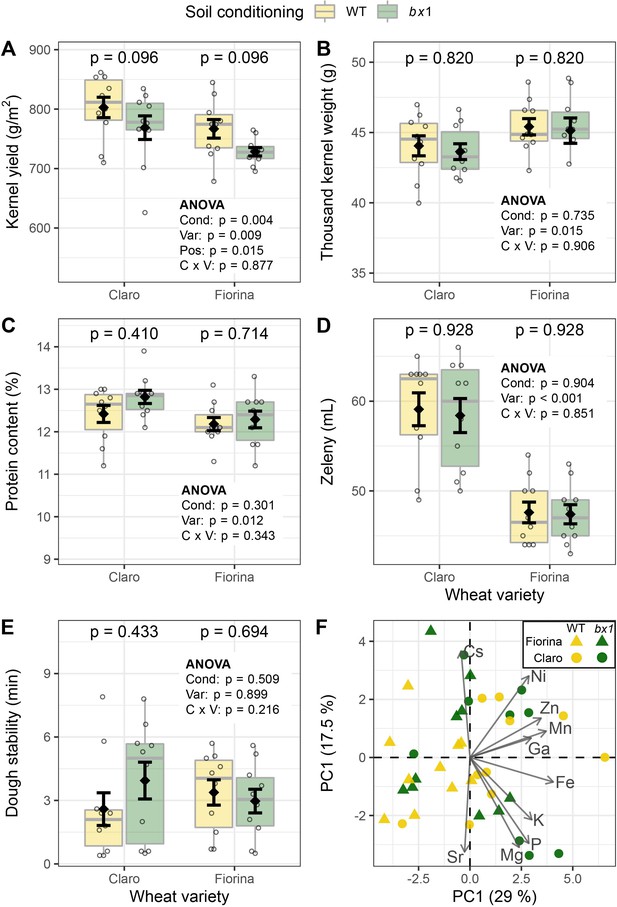
Benzoxazinoid soil conditioning increases wheat yield without compromising grain quality.
(A) Yield of two wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize. Kernel quality measures included (B) thousand kernel weight, (C) kernel protein content, (D) Zeleny index (flour quality), (E) dough stability, and (F) PCA of kernel micronutrient composition. For (A–E) means ±SE, boxplots, and individual datapoints are shown (n=10). ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. (F) reports the first two axes of the micronutrient PCA, including individual samples and the contribution of the 10 elements explaining most of the variation in the dataset (arrow length denotes relative contribution). Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Pos: position on the field. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
-
Figure 6—source data 1
Data of phenotypes shown in Figure 6, Figure 6—figure supplement 1 and Figure 6—figure supplement 2.
- https://cdn.elifesciences.org/articles/84988/elife-84988-fig6-data1-v1.xlsx
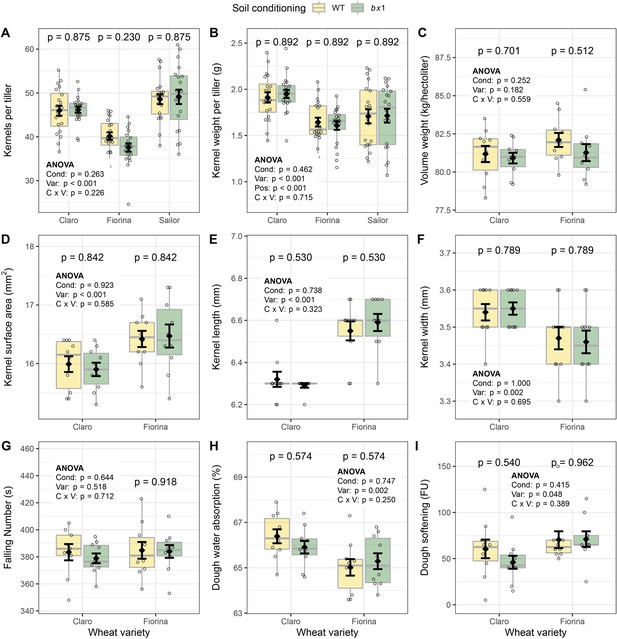
Benzoxazinoid soil conditioning does not affect wheat kernel measurements and baking quality.
(A) Kernels per tiller of three wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize (n=10). (B) Kernel weight per tiller (n=10), (C) kernel volume per weight (n=10), (D) kernel surface area (n=10), (E) kernel length (n=10), (F) kernel width (n=10), (G) falling number (flour quality, n=10), (H) flour water absorption (n=10), and (I) dough softening (n=9–10). Means ±SE, boxplots, and individual datapoints are shown. ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Pos: position on the field. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.

Benzoxazinoid soil conditioning does not affect micronutrient concentrations in wheat kernels.
Concentration of individual elements in kernels of two wheat varieties growing in soils previously conditioned with wild type (WT) or benzoxazinoid-deficient bx1 mutant maize (n=8–10). Means ±SE, boxplots, and individual datapoints are shown. ANOVA tables and pairwise comparisons within each wheat variety (FDR-corrected p values) are included. Same data as shown in PCA Figure 6F. Cond: soil conditioning (WT or bx1). Var: wheat variety. ‘C x V’: interaction between conditioning and wheat variety. Note that the minimum values of the y-axes were set to a value greater than zero for clearer visualization of treatment differences.
Additional files
-
Supplementary file 1
Specifications to library preparation for sequencing.
- https://cdn.elifesciences.org/articles/84988/elife-84988-supp1-v1.xlsx
-
Supplementary file 2
Barcode-to-sample assignments and meta data of microbiome analysis.
- https://cdn.elifesciences.org/articles/84988/elife-84988-supp2-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/84988/elife-84988-mdarchecklist1-v1.docx





