Coordinated molecular and ecological adaptations underlie a highly successful parasitoid
Figures
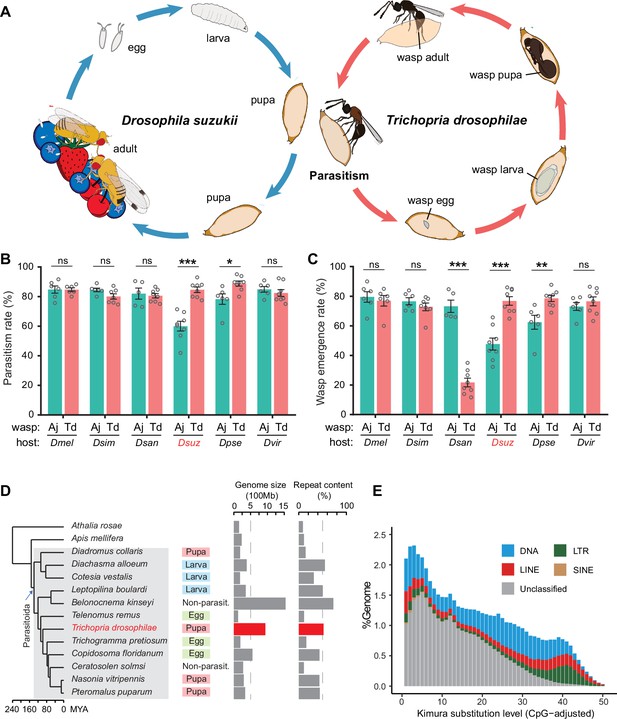
Chromosome-level assembly of a generalist drosophilid parasitoid genome.
(A) The life cycle of T. drosophilae (parasitoid) and Drosophila (host). T. drosophila (Td) is a pupal parasitoid that deposits eggs into Drosophila host pupae. As a generalist parasitoid, Td can successfully parasitize a variety of Drosophila species, including the well-known invasive pest D. suzukii. (B) and (C) The comparison of parasitization performance between Td and A. japonica (Aj). The parasitism rates (B) and wasp emergence rates (C) in six Drosophila species parasitized by Aj and Td. At least five biological replicates were performed. Data represent the mean ± SEM. Significance was analysed by two-way ANOVA with Sidak’s multiple comparisons test (ns, not significant; *p<0.05; **p<0.01; ***p<0.001). Dmel, D. melanogaster; Dsim, D. simulans; Dsan, D. santomea; Dsuz, D. suzukii; Dpse, D. pseudoobscura; Dvir, D. virilis. (D) Genome size and repeat content of representative parasitoid species in the context of phylogeny. Parasitoid species were selected based on the presence of high-quality genome reference and the representativeness of the group. The corresponding host life stage is shown in the coloured box. (E) The age and relative abundance of repeat element classes in Td genome. Kimura divergence from the consensus was estimated to indicate the burst time of repeats. See Figure 1—figure supplement 2 for the plots of all involved parasitoid species.
-
Figure 1—source data 1
Related to Figure 1B.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig1-data1-v1.xlsx
-
Figure 1—source data 2
Related to Figure 1C.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig1-data2-v1.xlsx
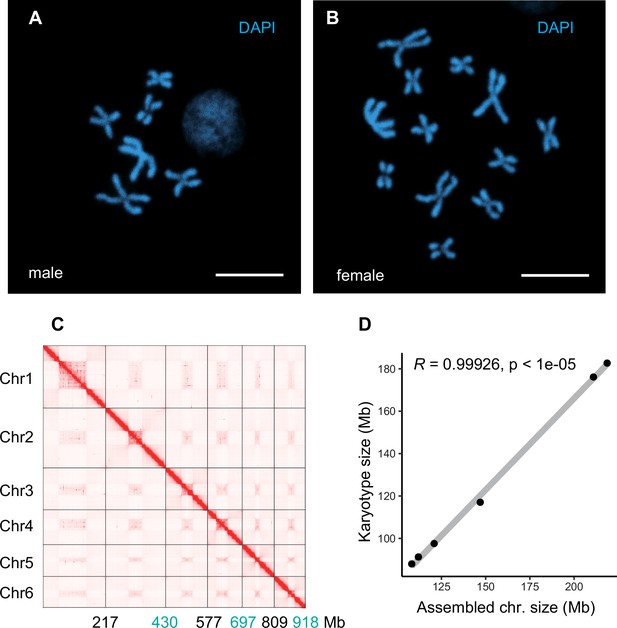
Chromosome features of Trichopria drosophilae (Td).
(A) Chromosome staining of male Td haploid chromosome (n=6). Scale bars: 10 μm. (B) Chromosome staining of female Td diploid chromosome sets (2n=12). Scale bars: 10 μm. (C) The heatmap of Hi-C all-by-all interaction among six chromosomes of Td. The y-axis refers each chromosome. The x-axis refers every chromosome with assembly scaffold length. The frequency of Hi-C interaction links is represented by the colour, which ranges from white (low) to red (high). The map indicates that intrachromosome interactions (red blocks in the diagonal) were stronger than interchromosome interactions (white blocks). (D) Correlation between estimated chromosome sizes (in Mb) based on karyotype images and assembled scaffolds. The values on y-axis indicate the estimated karyotype size of a chromosome, which is calculated based on collected the chromosome size and the length of chromosome in karyotype images of closely related species (Aphelinus varipes). Then we used them as a reference to obtain the estimated karyotype chromosome sizes based on the length of chromosomes in the karyotype images of Td. Each dot indicates a single chromosome. The correlation coefficient is 0.99926, indicating near good correlation between chromosomal scaffold length and karyotypically determined chromosome length. Raw data was provided in Supplementary file 8.
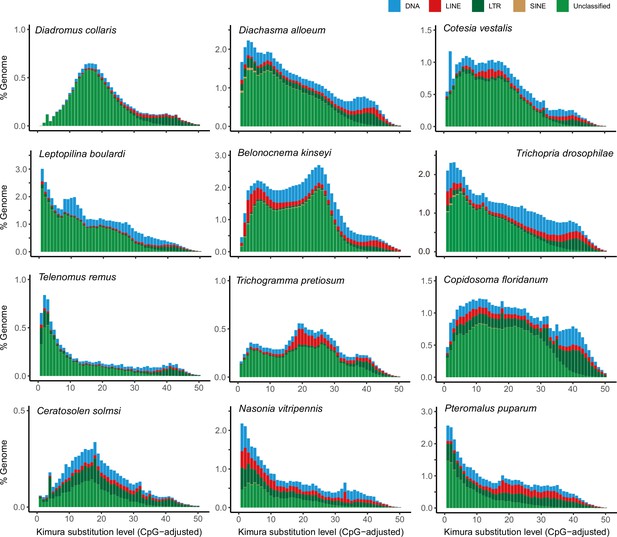
Distribution of repeat content among representative parasitoid species.
The age and relative abundance of repeat element classes across representative parasitoid genomes. The CpG-adjusted Kimura divergence from the consensus was estimated to indicate the time of duplication.
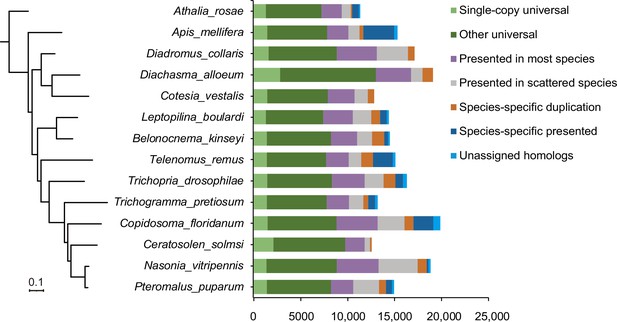
Phylogeny and ortholog content across representative parasitoid species.
The phylogeny on the left is a maximum-likelihood phylogenetic tree based 1246 single-copy orthologs across 12 representative species with high-quality reference genomes as well we two related hymenopteran species. The assignment of ortholog contents is shown as histograms on the right.
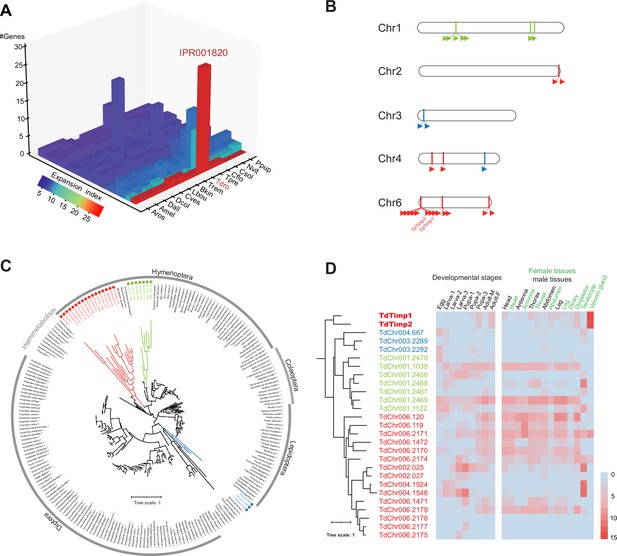
Genomic distribution and expression profiling of Timps in Trichopria drosophilae (Td).
(A) Top 20 expanded functional domains in Td genome in comparison to other parasitoids. Species abbreviations correspond to those in Figure 1D. (B) Genomic locations of characterized metalloproteinases (Timp) genes on Td chromosomes. (C) Maximum-likelihood phylogenetic tree across all identified Timp in insects. The copies in Td are highlighted in colour. The colours also correspond to those on (B) and (D). (D) Transcriptome expression profiles of Timp genes across different developmental stages and representative tissues. The phylogeny is based on the expression pattern.
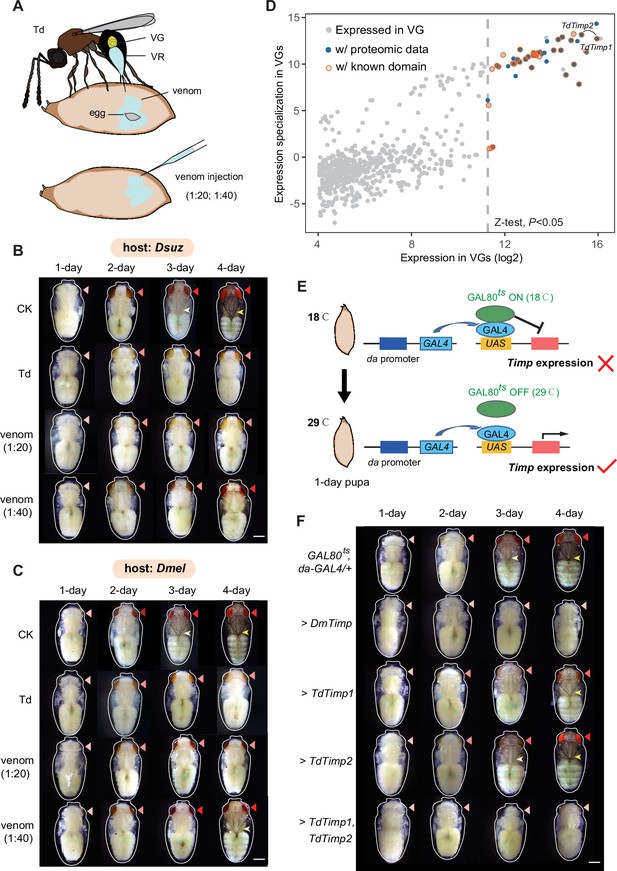
Trichopria drosophilae (Td) venom glands recruit a family of metalloproteinases (Timp) as venom proteins to arrest the host development.
(A) Schematic diagram of Td parasitization and venom injection into Drosophila host pupa. Wasp venom is produced in the venom gland (VG) and stored in the venom reservoir (VR). (B) and (C) Development of D. suzukii (B) and D. melanogaster (C) pupae treated by Td parasitization and venom injections. One-day-old host pupae were either parasitized by Td or injected with wasp venom at 1:20 and 1:40 dilutions or PBS (CK). Fly eyes are marked by red arrowheads, and bristles are marked by yellow arrowheads. Here, darker red eye colour and bristle appearance represent the older development of host pupae. Scale bar: 400 μm. More than 40 host pupae were examined for each group. (D) Identification and annotation of venom proteins (VPs). The expression value in the VGs and its specialization level are presented on the x-axis and y-axis, respectively. The specialized index was estimated by the ratio of expression in VGs to the mean value of any other tissues and developmental stages (Supplementary file 3). (E) Schematic illustration of the temperature-sensitive conditional GAL4/GAL80ts system to drive UAS-Timp expression in host pupae. At the permissive temperature (18 °C), binding of GAL80ts to GAL4 prevented the transcription of Timp genes. After shifting to 29 °C, GAL80ts was inactivated, allowing da-GAL4 to ubiquitously activate the transcription of Timp genes. (F) Development of D. melanogaster pupae after ectopic expression of D. melanogaster Timp (DmTimp) and TdTimps. One-day-old D. melanogaster pupae were transferred from 18to 29°C using the GAL4/GAL80ts system to ubiquitously overexpress Timp genes in host pupae. Fly eyes are marked by red arrowheads, and bristles are marked by yellow arrowheads. Here, darker red eye colour and bristle appearance represent the older development of host pupae. The outline around each representative image refers to the pupa case. Scale bar: 400 μm. More than 40 host pupae were examined for each group.
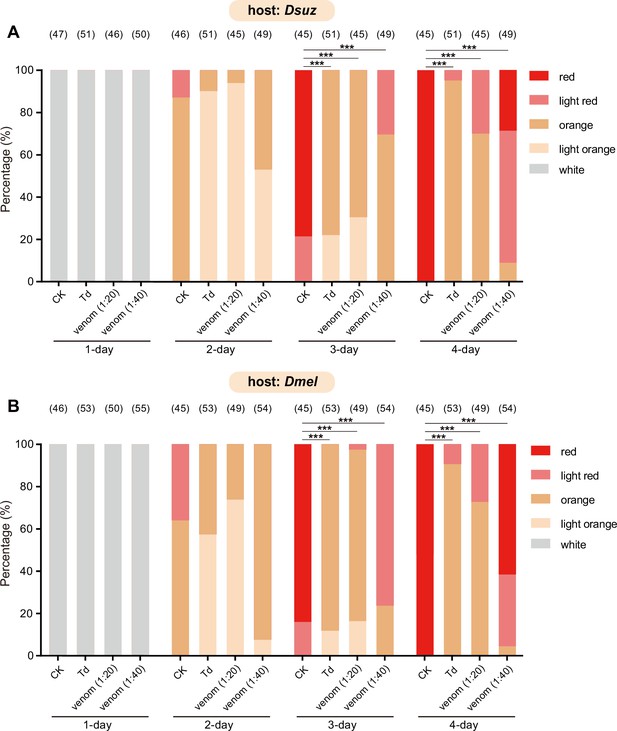
Characterization of the developmental delay phenotype in Figure 3B (A) and Figure 3C (B).
The eye colours of Drosophila hosts were divided into ive levels from white (young) to red (old), including white, light orange, orange, light red, and red. The percentage of D. suzukii (A) and D. melanogaster (B) pupae with different eye colours were quantified. Significance of the proportion of red eye pupae was analyzed by Fisher’s exact test (***p<0.001). The n value of each experiment was provided above the column.
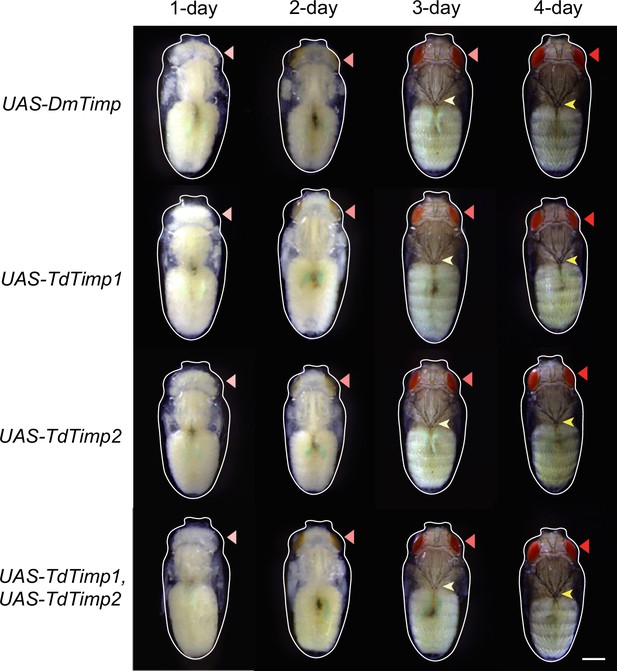
Development of D. melanogaster UAS-controls.
The UAS-controls for Figure 3F, including UAS-DmTimp, UAS-TdTimp1, UAS-TdTimp2, and UAS-TdTimp1 +TdTimp2. Fly eyes are marked by red arrowheads, and bristles are marked by yellow arrowheads. The outline around each animal refers to the pupa case. Scale bar: 400 μm.
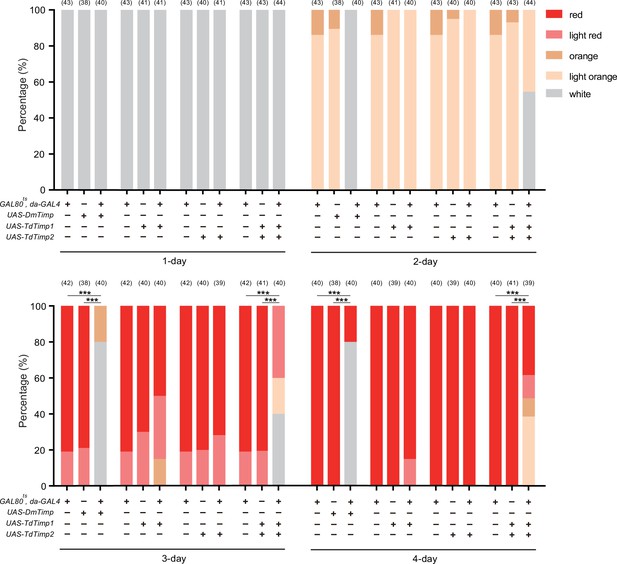
Characterization of the developmental delay phenotype in Figure 3F and Figure 3—figure supplement 2.
The eye colours of D. melanogaster were divided into 5 levels from white (young) to red (old), including white, light orange, orange, light red, and red. The percentage of D. melanogaster pupae with different eye colours were quantified. Significance of the proportion of red eye pupae was analyzed by Fisher’s exact test (***p<0.001). The n value of each experiment was provided above the column.
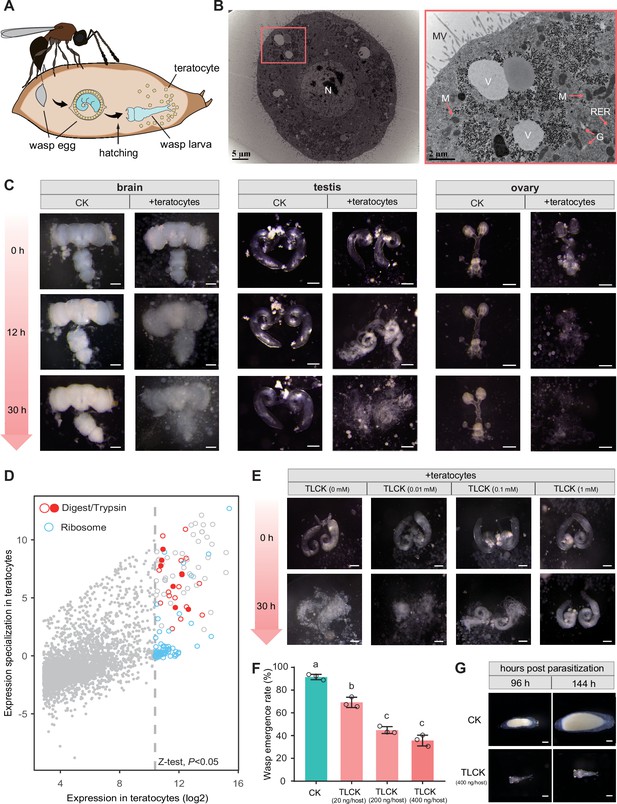
Trichopria drosophilae (Td) teratocytes secret trypsin proteins to digest host tissues.
(A) Schematic diagram of teratocytes in Td-parasitized Drosophila hosts. Teratocytes are derived from the serosal membrane during Td egg hatching. (B) Transmission electron microscopy of Td teratocytes. Details of the micrograph (red box) showing the perinuclear region containing abundant rough endoplasmic reticulum (RER), numerous mitochondria (M), Golgi apparatus (G), and vesicles (V). Note that microvilli (MV) are obvious on the external surface of the teratocyte membrane, and the nucleus (N) is relatively large. Scale bars: shown in the images. (C) D. suzukii host tissues, including the brain, testis, and ovary, were cocultured with teratocytes derived from a single parasitized host. Host tissues cocultured with Schneider’s medium only were used as controls (CK). The status of the host tissues was recorded after 0 hr, 12 hr, and 30 hr of incubation. Scale bars: 200 μm. More than 20 tissues were examined for each group. (D) Identification of effecting components in teratocytes. The expression value in the teratocytes and associated specialized level to other tissues and developmental stages are presented on the x-axis and y-axis, respectively. The specialized index was calculated as the ratio of expression value in teratocytes to the mean value of other tissues and developmental stages (Supplementary file 4). (E) Different concentrations of a trypsin inhibitor, TLCK, were added to the mixtures of D. suzukii testes and teratocytes. The status of the host tissues was recorded after 0 hr and 30 hr of incubation. Scale bars: 200 μm. More than 20 testes were examined for each group. (F) The wasp emergence rate in D. suzukii parasitized by Td after injection of different amounts of TLCK (20 ng, 200 ng, 400 ng per host pupa). ddH2O injection was used as a control (CK). Three biological replicates were performed. Data represent the mean ± SEM. Significance was analysed by one-way ANOVA with Sidak’s multiple comparisons test. Different letters indicate statistically significant differences (p<0.05). (G) Representative images of Td larvae in CK and TLCK-injected hosts after 96 hr and 144 hr parasitization. Scale bars: 200 μm. More than 20 larvae were examined for each group.
-
Figure 4—source data 1
Related to Figure 4F.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig4-data1-v1.xlsx
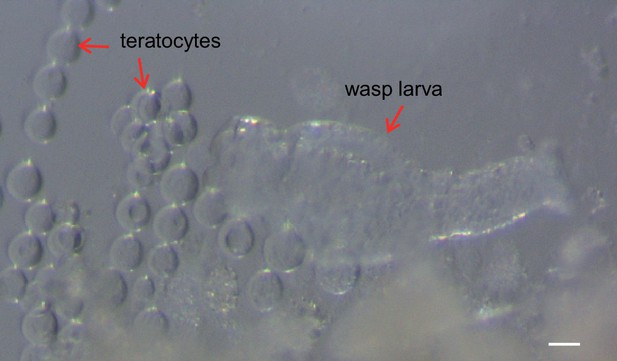
The teratocytes are released during Trichopria drosophilae (Td) egg hatching.
A Td egg was dissected and cultured in Schneider’s medium (Thermo Fisher Scientific, Cat#21720001). When the egg hatched into a larva in the culture medium, it released teratocytes as well. Scale bar: 100 μm.
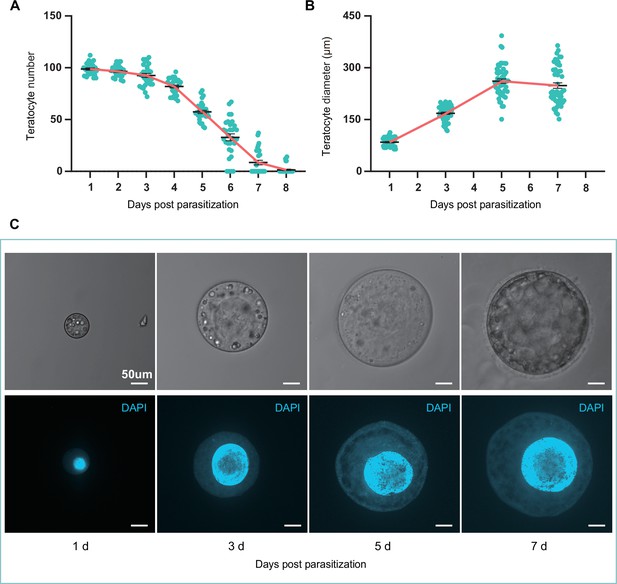
The size and number of teratocytes in parasitized hosts.
(A) The teratocyte numbers in single D. suzukii host of different days post Trichopria drosophilae (Td) parasitization. n=31 for each group. (B) The teratocyte diameters in D. suzukii host of different days post Td parasitization. Left to right, n=52, 53, 52 and 50 teratocytes. (C) The Light microscopy images and DAPI staining images of teratocytes in D. suzukii host of different days post Td parasitization. Scale bars: 50 μm. At least 20 teratocytes were examined for each group.
-
Figure 4—figure supplement 2—source data 1
Related to Figure 4—figure supplement 2A.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig4-figsupp2-data1-v1.xlsx
-
Figure 4—figure supplement 2—source data 2
Related to Figure 4—figure supplement 2B.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig4-figsupp2-data2-v1.xlsx
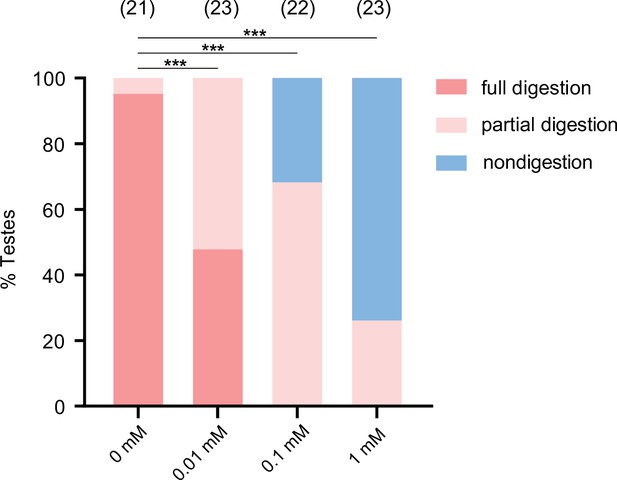
A trypsin inhibitor, TLCK, impairs the degradation function of teratocyte.
The percentage of host tissue at different digestion status in Figure 4E after incubation with a trypsin inhibitor, TLCK. Significance of the proportion of full digestion was analyzed by Fisher’s exact test (***p<0.001). The n value of each experiment was provided above the column.
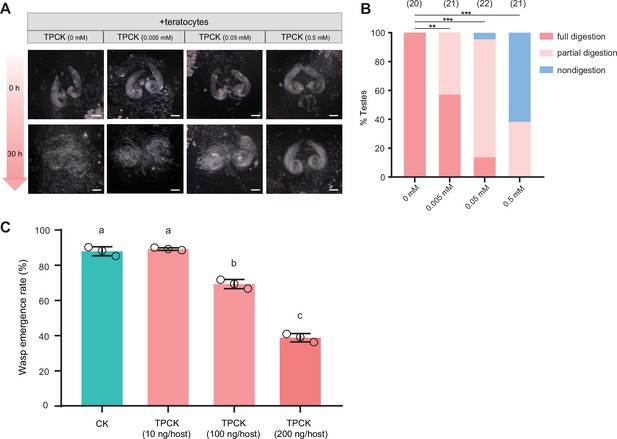
A trypsin inhibitor, TPCK, impairs the degradation function of teratocyte.
(A) Different concentrations of a trypsin inhibitor, TPCK, were added to the mixtures of D. suzukii testes and teratocytes. The status of the host tissues were recorded after 0 hr, and 30 hr incubation. Scale bars: 200 μm. At least 20 testes were examined for each group. (B) The percentage of host tissue at different digestion status in (A). Significance of the proportion of full digestion was analyzed by Fisher’s exact test (**p<0.01, ***p<0.001). The n value of each experiment was provided above the column. (C) The wasp emergence rate in D. suzukii parasitized by Td after injection of different amounts of TPCK (10 ng, 100 ng, 200 ng per host pupa). DMSO injection was used as a control (CK). Three biological replicates were performed. Data represent the mean ± SEM. Significance was analyzed by one-way ANOVA with Sidak’s multiple comparisons test. Different letters indicate statistically significant differences (p<0.05).
-
Figure 4—figure supplement 4—source data 1
Related to Figure 4—figure supplement 4C.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig4-figsupp4-data1-v1.xlsx
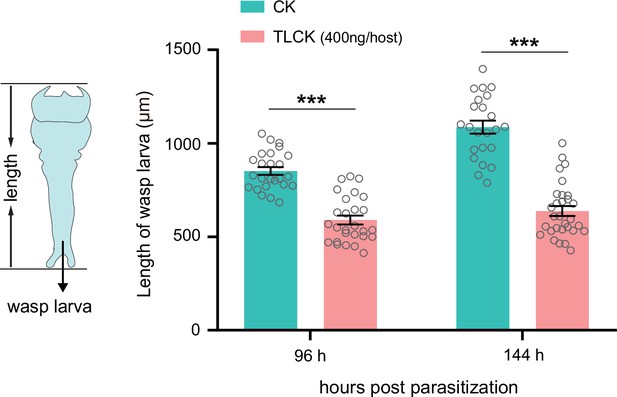
Inhibition of trypsins impairs the development of wasp larvae.
The length of the Td larvae in CK (H2O) and TLCK-injected hosts after 96 hr and 144 hr parasitization. Left to right: n=25, 24, 28, and 30 biologically independent larvae. Data represent the mean ± SEM. Significance was determined by two-way ANOVA with Sidak’s multiple comparisons test (***p<0.001).
-
Figure 4—figure supplement 5—source data 1
Related to Figure 4—figure supplement 5.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig4-figsupp5-data1-v1.xlsx
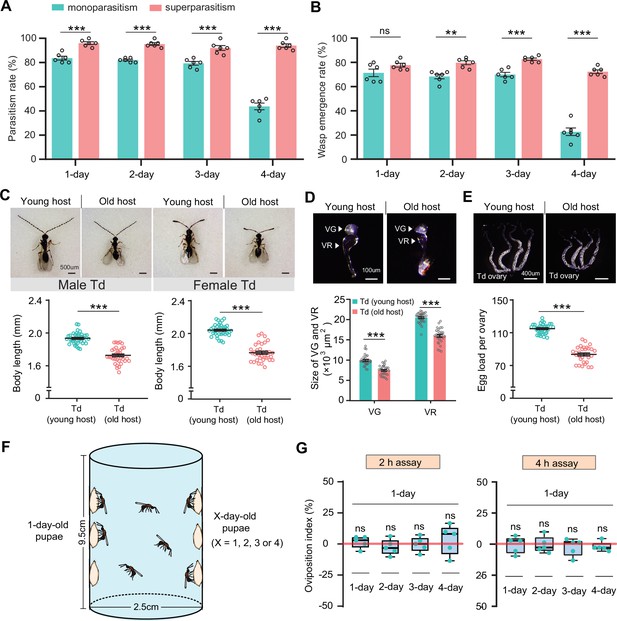
Superparasitism enhances Trichopria drosophilae (Td) parasitic efficiency.
(A) and (B) Parasitism rates (A) and wasp emergence rates (B) in 1- to 4-day-old D. suzukii pupae after monoparasitism or superparasitism by Td. Six biological replicates were performed. Data represent the mean ± SEM. Significance was analysed by two-way ANOVA with Sidak’s multiple comparisons test (ns, not significant; **p<0.01; ***p<0.001). (C) The images and body length of Td males and females emerged from young and old D. suzukii hosts. n=38 and 35 male wasps, n=39, and 34 female wasps, respectively. Data represent the mean ± SEM. Significance was determined by two-tailed unpaired Student’s t-tests (***p<0.001). Scale bar: 500 μm. (D) The size of the venom gland (VG) and venom reservoir (VR) of Td females emerged from young and old D. suzukii hosts. Left to right: n=33, 31, 33, and 31 wasps. Data represent the mean ± SEM. Significance was determined by two-tailed unpaired Student’s t-tests (***p<0.001). Scale bar: 100 μm. (E) The ovaries and egg load of Td females emerged from young and old D. suzukii hosts. n=33 and 30 wasps, respectively. Data represent the mean ± SEM. Significance was determined by two-tailed unpaired Student’s t-tests (***p<0.001). Scale bar: 400 μm. (F) Schematic diagram of the Td oviposition choice assays. One-day-old host pupae were placed on the left side, and the same number of X-day-old host pupae (X=1, 2, 3, or 4) were placed on the right side. Oviposition index = (N1 − Nx)/(N1 +Nx)×100%, where N1 is the total number of wasp eggs in the host pupae on the left and Nx is the total number of wasp eggs in the host pupae on the right. (G) Oviposition indices of Td females in different aged D. suzukii hosts from 2 hr and 4 hr assays. Five biological replicates were performed. Box plots represent the median (bold black line), quartiles (boxes), and minimum and maximum (whiskers). All oviposition indices were not significantly different from zero (bold red line), which represents no choice for Td egg laying. Deviation of the oviposition index against zero was tested with the Wilcoxon signed rank test (ns, not significant). Young host: 1-day-old pupae; old host: 4-day-old pupae.
-
Figure 5—source data 1
Related to Figure 5A.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-data1-v1.xlsx
-
Figure 5—source data 2
Related to Figure 5B.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-data2-v1.xlsx
-
Figure 5—source data 3
Related to Figure 5C.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-data3-v1.xlsx
-
Figure 5—source data 4
Related to Figure 5D.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-data4-v1.xlsx
-
Figure 5—source data 5
Related to Figure 5E.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-data5-v1.xlsx
-
Figure 5—source data 6
Related to Figure 5G.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-data6-v1.xlsx
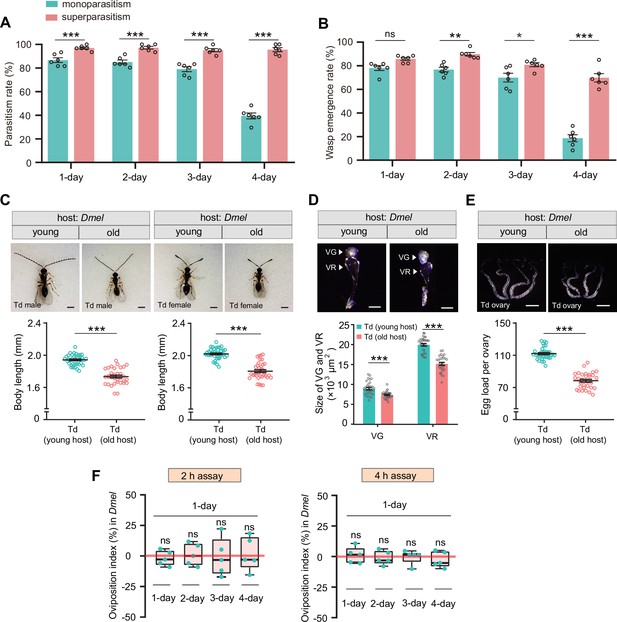
Superparasitism enhances Trichopria drosophilae (Td) parasitic efficiency on D. melanogaster.
(A) and (B) Parasitism rates (A) and wasp emergence rates (B) in 1- to 4-day-old D. melanogaster pupae after monoparasitism or superparasitism by Td. Six biological replicates were performed. Data represent the mean ± SEM. Significance was analyzed by two-way ANOVA with Sidak’s multiple comparisons test (ns, not significant; *p<0.05; **p<0.01; ***p<0.001). (C) The images and body length of Td males and females emerged from young and old D. melanogaster hosts. n=40 and 36 male wasps, n=35 and 33 female wasps, respectively. Data represent the mean ± SEM. Significance was determined by two-tailed unpaired Student’s t-tests (***p<0.001). Scale bar: 500 μm. (D) The size of venom gland (VG) and venom reservoir (VR) of Td females emerged from young and old D. melanogaster hosts. Left to right: n=32, 30, 32, and 30 wasps. Data represent the mean ± SEM. Significance was determined by two-tailed unpaired Student’s t-tests (***p<0.001). Scale bar: 100 μm. (E) The ovaries and egg load of Td females emerged from young and old D. melanogaster hosts. n=33 and 31 wasps, respectively. Data represent the mean ± SEM. Significance was determined by two-tailed unpaired Student’s t-tests (***p<0.001). Scale bar: 400 μm. (F) Oviposition indices of Td females in different aged D. melanogaster hosts from 2 hr and 4 hr assays. Five biological replicates were performed. Box plots represent the median (bold black line), quartiles (boxes), as well as the minimum and maximum (whiskers). All oviposition indices were not significantly different with zero (bold red line), which represents no choice for Td egg laying. Deviation of the oviposition index against zero was tested with Wilcoxon signed rank test (ns, not significant). Young host: 1-day-old pupae; old host: 4-day-old pupae.
-
Figure 5—figure supplement 1—source data 1
Related to Figure 5—figure supplement 1A.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-figsupp1-data1-v1.xlsx
-
Figure 5—figure supplement 1—source data 2
Related to Figure 5—figure supplement 1B.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-figsupp1-data2-v1.xlsx
-
Figure 5—figure supplement 1—source data 3
Related to Figure 5—figure supplement 1C.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-figsupp1-data3-v1.xlsx
-
Figure 5—figure supplement 1—source data 4
Related to Figure 5—figure supplement 1D.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-figsupp1-data4-v1.xlsx
-
Figure 5—figure supplement 1—source data 5
Related to Figure 5—figure supplement 1E.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-figsupp1-data5-v1.xlsx
-
Figure 5—figure supplement 1—source data 6
Related to Figure 5—figure supplement 1F.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig5-figsupp1-data6-v1.xlsx

The image of the host parasitized by two Trichopria drosophilae (Td) female wasps.
Two Td female wasps showed a collaboration to parasitize the same D. suzukii pupa. Scale bar: 500 μm.
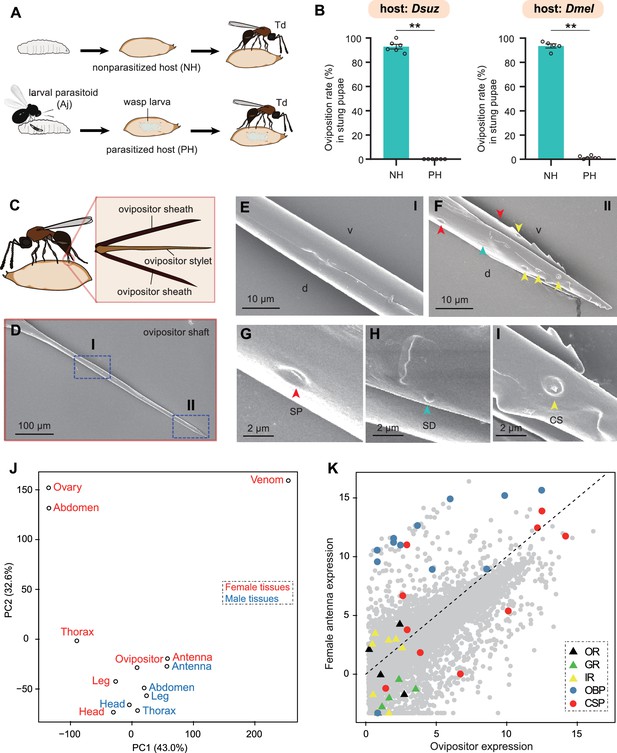
Trichopria drosophilae (Td) avoids interspecific competition through chemoreception in the ovipositor.
(A) Schematic diagram of the Td interspecific competition assay. PH, Aj-parasitized host; NH, non-parasitized host. (B) Oviposition rates of Td in NH and PH pupae of D. suzukii and D. melanogaster, respectively. At least five biological replicates were performed. Data represent the mean ± SEM. Significance was analysed by the Mann-Whitney U test (**p<0.01). (C) Schematic diagram Td ovipositor, which contains two ovipositor sheaths and one ovipositor stylet. (D) to (I) Transmission electron microscope micrograph of Td ovipositor stylet. Higher magnification micrographs show the proximal end (E), Region I in (D) and distal end (F, II in D) of the ovipositor stylet. Different types of sensilla (arrowheads) on ventral (v) and dorsal (d) valves are identified, including secretary pores (SP, red arrowhead) (G), surface-dome sensilla (SD, green arrowhead) (H) and coeloconic sensilla (CS, yellow arrowhead) (I). Scale bars are shown in the images. (J) Principal component analysis based on overall expression profiles across different tissues. (K) Expression profiles of Td genes in ovipositors and female antennae. Each dot indicates the log-transformed expression values (TPM) in ovipositors (x-axis) and in female antennae (y-axis).
-
Figure 6—source data 1
Related to Figure 6B.
- https://cdn.elifesciences.org/articles/94748/elife-94748-fig6-data1-v1.xlsx
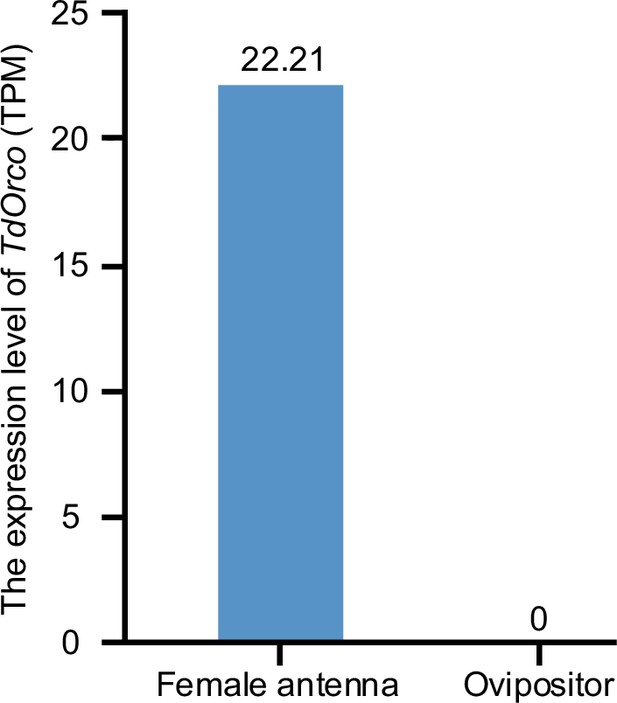
Transcriptome expression profile of Orco on female antenna and ovipositor of Trichopria drosophilae (Td).
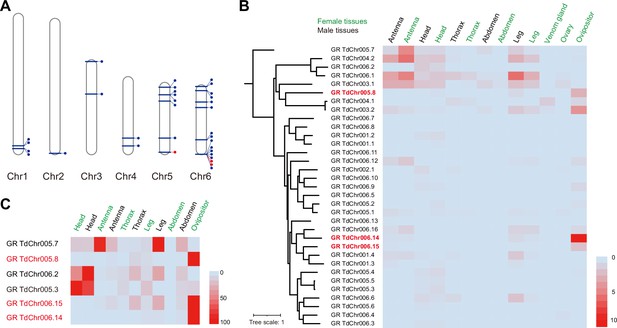
Genomic distribution and expression profiling of gustatory receptor genes in Trichopria drosophilae (Td).
(A) Genomic location of characterized gustatory receptor (GR) genes on Td chromosomes. (B) Transcriptome expression profiles of GR genes across representative tissues. The phylogeny is based on the expression pattern. (C) qPCR verification of selective GRs with high expression in ovipositors.
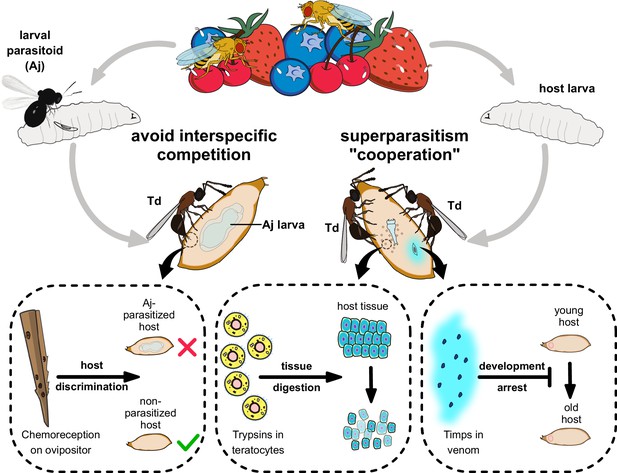
Proposed model of the molecular and ecological adaptions underlying the parasitic success in Trichopria drosophilae (Td).
Td is a solitary parasitoid that presents high levels of pupal parasitism to Drosophila species, including D. suzukii. Td allows intraspecific superparatism to increase the success of wasp emergence from hosts with the help of two weapons. Specifically, two Timp venom proteins (VPs) trigger the development arrest of Drosophila hosts, eventually leading to the maintenance of the hosts at a younger status, whereas trypsins in teratocytes contribute to the digestion of host tissues, providing suitable nutritional resources for the wasp larvae. Moreover, Td avoids interspecific competition with larval parasitoid species to extend their parasitic efficacy and mostly depends on chemoreception on the ovipositor.
Additional files
-
Supplementary file 1
Basic features of Trichopria drosophilae genome.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp1-v1.xlsx
-
Supplementary file 2
Top 20 expanded gene families in Trichopria drosophilae (Td).
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp2-v1.xlsx
-
Supplementary file 3
List of Trichopria drosophilae (Td) venom protein genes.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp3-v1.xlsx
-
Supplementary file 4
List of Trichopria drosophilae (Td) teratocyte genes.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp4-v1.xlsx
-
Supplementary file 5
List of Trichopria drosophilae (Td) chemoreception genes.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp5-v1.xlsx
-
Supplementary file 6
Primer sequences in this study.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp6-v1.xlsx
-
Supplementary file 7
Transcriptome sequencing data in this study.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp7-v1.xlsx
-
Supplementary file 8
Raw data in Figure 1—figure supplement 1D.
- https://cdn.elifesciences.org/articles/94748/elife-94748-supp8-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/94748/elife-94748-mdarchecklist1-v1.docx





