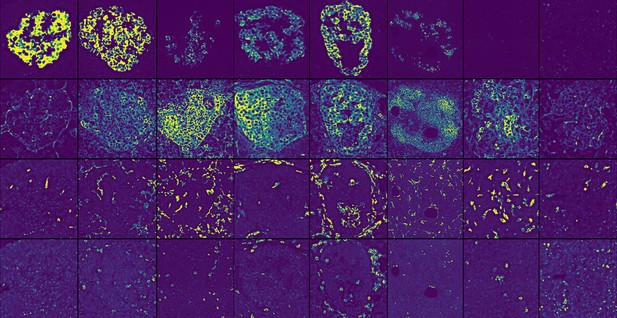
Pancreatic islets from individuals with autoimmune type 1 diabetes at different stages of insulitis. Each row depicts a different protein that is used to identify an important cell type or biological process. From top to bottom: Proinsulin labels β-cells, HLA-ABC labels cells prone to interacting with T cells, CD163 labels macrophages, and CD8 labels a type of T cell. Image credit: Barlow et al. (CC BY4.0)
When someone has type 1 diabetes, their immune system mistakenly targets and destroys β-cells in the pancreas, which produce insulin, the hormone that helps bring down sugar levels in the blood after we eat. Despite advances in treatment, most people with type 1 diabetes will depend on insulin for their entire lives.
T cells are a type of immune cell involved in type 1 diabetes. These cells infiltrate the pancreatic islets, the structures where β-cells reside, to attack the β-cells. This process, called insulitis, is poorly understood, partly because obtaining tissue samples containing islets in the process of being infiltrated by T cells is extremely difficult.
Barlow et al. collaborated with the Network for Pancreatic Organ Donors with Diabetes to obtain pancreatic tissues from eight organ donors with type 1 diabetes, and two organ donors whose immune systems could recognize islets but who were not yet exhibiting diabetes symptoms.
Barlow et al. analysed 54 proteins in each tissue section and examined how inflammation progressed in islet cells and the surrounding pancreas to better understand insulitis. The researchers identified four types of insulitis, each defined by the types of T cells present. The nature of the T cells in islets is important because it may affect how fast type 1 diabetes progresses. Although Barlow et al. did not examine enough cases to establish if a correlation exists between the types of insulitis and disease progression, this can be examined in future studies. They also found that, during insulitis, the blood vessels in the islets switched on a protein called IDO, possibly in response to T cells that infiltrate islets. IDO may temporarily protect the islets from the immune response as insulitis progresses, but it is insufficient to protect the β-cells. Barlow et al. further found aggregates of T cells mixed with B cells, another type of immune cell, in the pancreas tissue surrounding the islets. Given that B cells and T cells provide stimulatory signals to each other, these aggregates may promote inflammation and be a new therapeutic target.
Barlow et al. also wanted to understand why T cells target some islets more than others and why islet destruction is spatially organized. The team compared pancreatic areas with many inflamed islets to areas in the same donor where fewer islets were inflamed, finding that the cell composition differs. Interestingly, the types of cells that were different were not the same as those that were infiltrating islets. B cells, macrophages and T cells were the major cell types infiltrating islets, but the cells that varied outside islets were nerves, endothelial cells, and a third cell type that may have been innate lymphoid cells. These results indicate a crosstalk between the cells outside islets and those that infiltrate islets.
The results by Barlow et al. lay the groundwork for a better understanding of the biology underpinning how the immune system destroys β-cells in insulitis. The next steps would be to see if other cells in the islets can influence T cells and if diabetes could be delayed by inhibiting interactions between T cells and the relevant cells outside the islets. Moreover, it would be important to establish whether preserving IDO in endothelial cells could delay diabetes symptoms.




