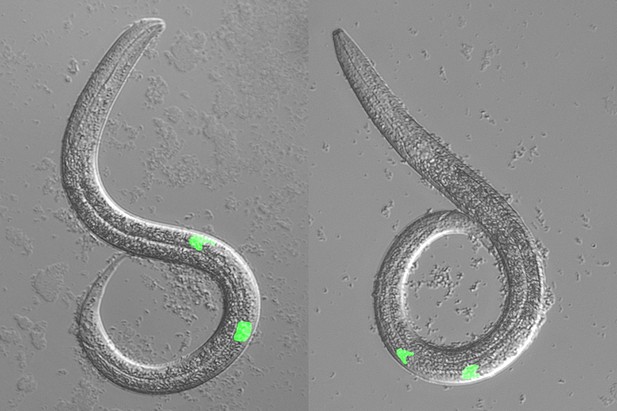
Image comparing a wild-type (left) and a mutant C. elegans (right) that has mutations in the genes for LIN-17, EGL-20 and CWN-2. Loss of these proteins reverses the polarity of the cell Z1, resulting in both distal tip cells (shown in green) migrating to the posterior of the worm, disrupting the mirror-symmetry of the gonads. Image credit: Shuhei So (CC BY 4.0)
In humans and other animals, cells are organized into tissues and organs that each perform distinct roles in the body. Some organs and tissues have a mirror-symmetric structure, meaning they are divided into two halves that are exact reflections of one another. However, it is not fully understood how these types of structures form during development.
The formation of mirror-symmetric structures often relies on cell polarity, which is when the components of a cell – such as its structure, internal contents and functional regions – are unevenly distributed. In the nematode worm C. elegans, for example, their mirror-symmetric gonads (or sex organs) are formed by two polarized cells called Z1 and Z4.
Both Z1 and Z4 divide asymmetrically to produce two daughter cells with distinct concentrations of a particular transcription factor. For Z1, the daughter cell facing the anterior of the gonad has lower levels of the transcription factor than the posterior daughter cell, while the two cells generated by Z4 have the opposing mirror asymmetry. This polarity drives the production of two distal tip cells – one produced by the anterior daughter cell of Z1 and the other by the posterior daughter cell of Z4 – which migrate to opposite ends of the gonad.
A cell signaling pathway known as Wnt is crucial for establishing cell polarity in many species. However, a previous study found that C. elegans could still develop healthy gonads even when all five ligand proteins that activate the Wnt pathway were mutated. Here, So et al. reveal that these mutations can impact polarity, but only when LIN-17, the receptor for the Wnt ligands, is also mutated. Further experiments showed that LIN-17 can independently regulate cell polarity and compensate for the loss of Wnt signaling.
So et al. also identified three specific Wnt ligands – CWN-1, CWN-2 and EGL-20 – that collectively control the polarity of Z1 and Z4. Each protein has a distinct role: CWN-1 promotes Z1 and Z4 to have the same polarity, while CWN-2 induces the polarity of Z1 cells to reverse. EGL-20 then stops Z1 from regaining its original polarity and no longer mirroring the polarity of Z4.
These findings shed new light on how Wnt signaling contributes to the mirror-symmetric structure of C. elegans gonads. It is possible that these proteins play similar roles in other animals to help regulate how organs form.




