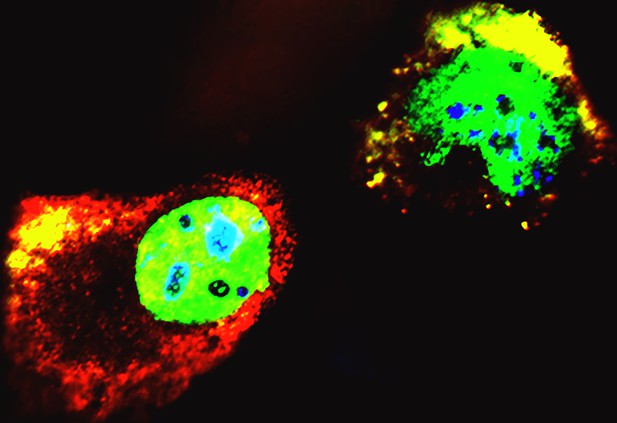
Isoforms of p53 forming aggregates with full-length p53 proteins. Image Credit: Zhao, Punga and Sanyal (CC BY 4.0)
Cancer is a deadly disease that continues to pose a major challenge to global healthcare systems. It arises when some cells acquire mutations that allow them to grow and divide uncontrollably. A key player for preventing this uncontrolled cell growth is the protein p53, which binds to DNA and regulates the activities of genes that determine whether a cell should grow, divide, or die.
Mutations in the DNA-binding domain of p53 can hinder its ability to regulate gene activity, thereby contributing to cancer. Additionally, shorter versions of p53 – known as p53 isoforms – can accumulate in the cell and coexist with the normal full-length protein. These isoforms sometimes lack critical components of the protein, whilst retaining other properties. For example, two p53 isoforms – called ∆133p53α and ∆160p53α – are missing parts of the DNA-binding domain but can still bind to full-length p53 protein. Here, Zhao, Punga and Sanyal used biochemical and cell biology approaches to investigate how the ∆133p53α and ∆160p53α isoforms affect the activity of full-length p53.
The team found that both isoforms can bind to full-length p53 protein and form tetramers, large molecules made up of four p53 sub-units. In addition, they found that the ∆133p53α and ∆160p53α isoforms can form aggregates with full-length p53. As a result, full-length p53 protein gets sequestered within these aggregates, which prevents them from binding to DNA and carrying out their role in gene expression and cell-death regulation.
These findings open up new avenues for cancer research, particularly in cancer patients who have truncated isoforms of the p53 protein. Developing new therapies that prevent shorter and normal full-length p53 protein from forming irregular aggregates may have the potential to prevent, or slow, cancer progression in these patients.




