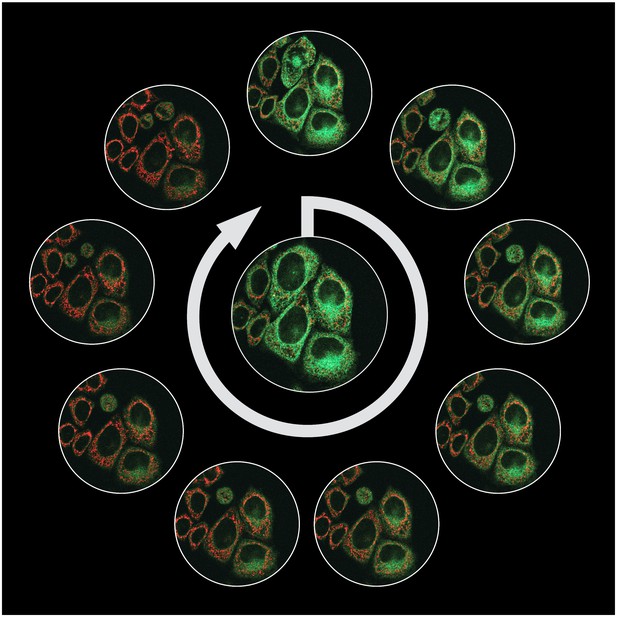
When a cell is starving for cholesterol (centre), it has high levels of the enzyme HMGCR (green), which is essential for the production of this fatty molecule. As cholesterol is re-supplied to the cell (with time increasing clockwise from the top of the image), HMGCR is marked for degradation and destroyed by the cell. In red are cellular sub-compartments called mitochondria. Image credit: Menzies, Volkmar et al. (CC BY 4.0)
Cholesterol is a fatty molecule that is essential for our health; for example, it is a component of the outer membrane that surrounds every cell in our body. Yet, it also has a reputation for clogging arteries and causing heart attacks and strokes. Our organism can adjust the amount of cholesterol it creates through an enzyme called HMGCR, which is found in all cells. Switching off HMGCR, for instance by taking drugs called statins, reduces the amount of cholesterol made by cells. To regulate the activity of HMGCR, the body uses proteins known as E3 ubiquitin ligases, which can label the enzyme for destruction. However, the identity of the ligases that target HMGCR is a matter of intense debate.
Here, Menzies, Volkmar et al. addressed this issue by using an approach called a genome-wide CRISPR forward genetic screen. First, HMGCR was marked inside the cells with a fluorescent tag to watch how its levels change in response to different amounts of cholesterol. Then, each gene in the cell was deleted, and the effects recorded. This allowed Menzies, Volkmar et al. to find the genes responsible for the rapid destruction of HMGCR.
The experiments revealed that the E3 ubiquitin ligases RNF145 and gp78 are independently responsible for the degradation of the majority of HMGCR, with a third ligase, Hrd1, getting involved if the first two are absent. In particular, RNF145 builds up when a cell is starved of cholesterol, but it immediately marks HMGCR for destruction once cholesterol becomes more abundant. This ligase can therefore both sense and respond to the amount of cholesterol in a cell, making it a perfect candidate for regulating HMGCR based on what the body needs.
Identifying the proteins that adjust the levels of HMGCR sheds light on how a cell controls the amount of cholesterol it creates. This knowledge could be relevant in the fight against the health problems associated with this molecule.




