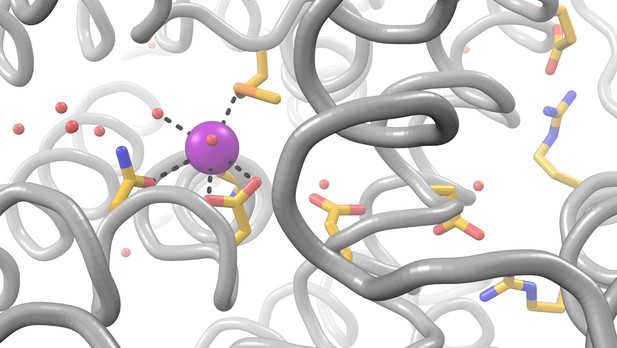
An Nramp transporter uses strategically positioned chemical groups (colored sticks) to capture a metal ion (magenta) and provide a parallel pathway for proton transport. Image credit:
Cells use transport proteins embedded in their membrane to acquire many of the nutrients they need to survive and grow. Different transport proteins transport different nutrients; for example, the Nramp transporters move transition metal ions across cell membranes. Nramps are found in a wide range of organisms. Bacteria use them to acquire the metals they need during the course of an infection, and humans rely on Nramps to absorb iron from food. Nramps can also transport hydrogen ions (known as protons).
Understanding how the structure of an Nramp transporter changes as it transports metal ions and protons can help researchers to understand how it works. These structures can be studied using a technique called X-ray crystallography, which captures snapshots of the proteins at different stages of their task.
Bozzi, Zimanyi et al. used X-ray crystallography to study the structures of an Nramp transporter from the bacterium Deinococcus radiodurans. The results reveal four of the shapes that the Nramp transporter takes on at different stages in its transport process, including the first structure to show an Nramp binding to a metal ion from the outside of the cell. Taken together, the structures suggest a new transport mechanism that has not been seen in previously studied transport proteins with similar structures. An unexpected feature of this mechanism is that Nramps transport metal ions and protons along different pathways.
Studying the transport mechanisms used by Nramp transporters will help researchers to understand how cells maintain appropriate levels of metal ions, an important component of human health. The mechanisms of relatively few transport proteins are understood at a structural level, yet many share common origins and have shared characteristics. Understanding how Nramps work could therefore help us to understand how wider classes of transporters work as well.




