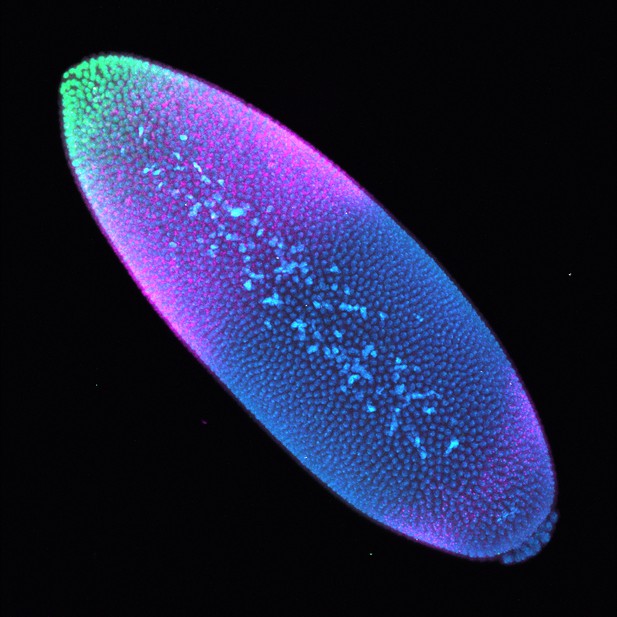
In the front half of the fruit fly embryo, the transcription factor Bicoid (green) controls expression of the developmental gene Hunchback (red), through a key enhancer called Hunchback P2. Image credit: Park et al. (CC BY 4.0)
Building an organism from scratch requires genes to be switched on or off at precisely the right time, in the right place, and at the right level. Enhancers are stretches of DNA that work as switches to turn on target genes. For instance, in the front part of fruit fly embryos, the P2 enhancer switches on a gene called Hunchback, which is crucial for development.
A number of molecular actors, including proteins called transcription factors, work together to turn on genes by interacting with enhancers. Genes like Hunchback can turn on suddenly, even though they are controlled by transcription factors whose levels are changing gradually: in other words, if Hunchback were controlled by a light switch with a dimmer, the light would suddenly come on as the dimmer was gradually moved up. For enhancers, the question is how transcription factors interact with DNA to convert a gradual input into an abrupt, sharp switch. A commonly accepted view is that Hunchback is turned on when molecules of a transcription factor called Bicoid help each other to bind to multiple binding sites on the P2 enhancer.
Park et al. investigated this mechanism by examining how the Hunchback gene responded to changes in the sequence of the P2 enhancer, and to changes in the levels of regulatory proteins that bind to it. The resulting observations were then compared to mathematical models that simulate turning on Hunchback under different conditions. The experiments revealed that, in fact, switching on Hunchback requires more than Bicoid proteins helping each other to bind on the P2 enhancers. Molecules other than Bicoid were also needed, and the cell also potentially had to burn energy.
Variations in the sequence of enhancers are linked to evolution of new species but also to problems in development or even diseases such as cancer. Understanding precisely how these sequences turn on genes will give us insight into which types of changes are important for disease and evolution.




