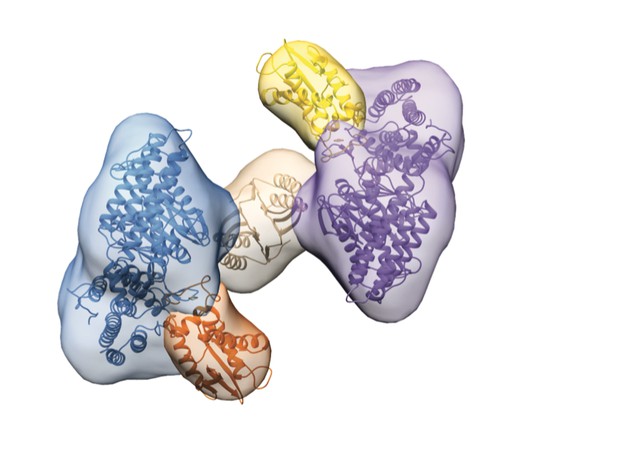
Architecture of a Cas4-Cas1-Cas2 complex. Image credit: Lee et al. (CC BY 4.0)
Many people have now heard of CRISPR, or CRISPR-Cas9, as a gene editing technology. Yet CRISPR evolved in bacteria to protect them against viral infections. While parts of the CRISPR system are now being widely used, the research community still does not know everything about how the system operates in its natural setting.
In bacteria, CRISPR protects against infection by making lasting records of viruses a cell has encountered. It cuts short sections from the viral DNA and keeps them as a way to fight the virus if it ever returns. The key proteins in collecting and storing the virus DNA are called Cas1, Cas2 and Cas4. Previous work suggests that Cas4 is important for cutting suitable lengths of DNA for storage. Yet, how Cas4, Cas1 and Cas2 work together to select, cut and store DNA is not well studied.
Lee et al. have now used electron microscopy to examine how Cas1, Cas2 and Cas4 cooperate in the CRISPR system. The proteins studied came from bacteria called Bacillus halodurans. The structure revealed direct links between the Cas1 and Cas4 proteins that likely help to ensure these proteins are coordinated correctly to cut and store the DNA sections. Specifically, it showed that two Cas4 proteins interact with the two key active sites of Cas1. The findings also highlight that Cas4 cuts DNA at specific locations to make sure the resulting DNA sections are suitable for CRISPR protection.
The close association between Cas1 and Cas4 could be a critical aspect of the reliability of the CRISPR system in protecting bacteria from viruses. There are more bacteria on Earth than any other living thing. Understanding their biology has wide ranging environmental, health and bioengineering applications. In addition, learning more about the CRISPR system could further expand its potential to drive revolutionary biotechnology tools derived from these bacterial immune systems.




