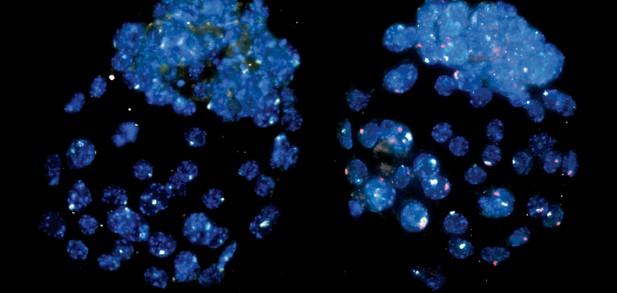
Early mouse embryos with (left) and without (right) maternally-derived EED protein. White indicates Inactivation of the X-chromosome from the father, and red indicates inactivation of the X-chromosome from the mother. Image credit: Harris, Cloutier et al. (CC BY 4.0)
Almost every one of our cells, with a few exceptions, contains the complete set of genes needed to build and maintain the human body. Yet, not all of these genes are active in every cell. Instead, some genes are tagged for activation, while others are silenced. These changes do not alter the genetic code, only how it is read by the cell, and are collectively referred to as epigenetics.
Female mammals have two X-chromosomes compared to males' one. As such, females will silence one of those chromosomes to avoid getting a double-dose from those genes located on the X-chromosome. This epigenetic process is called X-chromosome inactivation, and it lasts for the life of the animal.
Epigenetic information can also be passed on to future generations. In early female embryos of mice, for example, it is always the X-chromosome inherited from the father that is suppressed, which suggests that the instructions for which X-chromosome to inactivate must have come from the parents.
Harris, Cloutier et al. set out to dissect the mechanics of the specialised form of X-chromosome inactivation seen in female embryos of mice, which is known as imprinted X-inactivation. A protein called EED was suspected to play a key role. Embryos inherit EED protein from the mother's egg, so it was reasoned that this protein may be the epigenetic link between the generations. The cascade of epigenetic events leading to imprinted X-inactivation in the early embryo has been well-defined, but the role of maternal EED was yet to be tested.
The experiments showed that the mother's EED protein was needed to silence the father's X-chromosome in female mouse embryos. Without EED from the mother's egg, early embryos failed to initiate imprinted X-inactivation and reverted instead to random X-inactivation, where either X-chromosome is chosen for silencing in female cells. This pattern resembles what happens in early human embryos, which are unable to undergo imprinted X-inactivation because a woman's eggs lack the EED protein.
Together these new findings trace the passage of epigenetic information from parent to offspring at the molecular level. With evidence like this, scientists can better understand mechanisms of non-genetic inheritance more broadly, including from parent to offspring.




