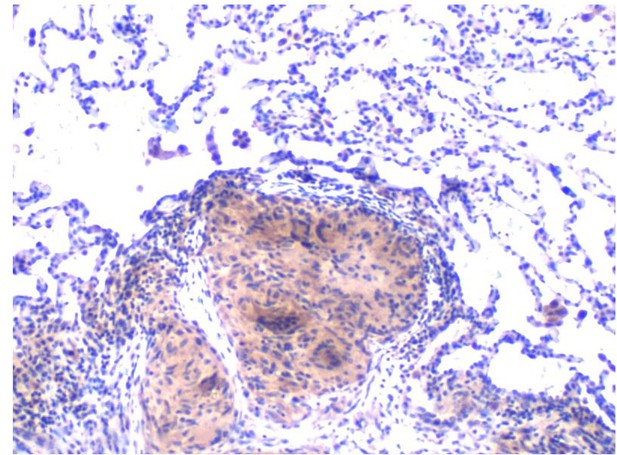
Lung biopsy of a sarcoidosis patient showing a classic sarcoidosis granuloma (pink) surrounded by normal lung tissue. Image credit: Lobelia Samavati (CC BY 4.0)
Sarcoidosis is a rare disease that is characterized by the formation of small lumps known as granulomas inside the body. These lumps are made up of clusters of immune cells, and are commonly found in the skin, lung or eye. Other organs of the body can also be affected, and symptoms will vary depending on where in the body lumps form. There is currently no specific treatment for sarcoidosis, as the direct cause of the disease is unknown. The disease is often treated with drugs that suppress the immune system. However, this type of treatment can lead to significant side effects and patients will respond to these drugs in different ways.
Patients with sarcoidosis have a heightened immune response to microbes that can cause infections, and rather than providing protection, this heightened response causes damage and inflammation to the body’s organs. Now, Talreja et al. have identified which genes and proteins control this inflammatory response in immune cells from the lungs and blood of sarcoidosis patients.
Immune cells in the lungs of sarcoidosis patients were found to have higher levels of hypoxia inducible factor (HIF) – a gene-regulating protein that controls the uptake and metabolism of oxygen in mammals. In addition, lung tissue affected with granulomas also expressed increased levels of a specific version of HIF known as HIF-1. Talreja et al. showed that the increased expression of HIF in the immune cells of sarcoidosis patients was mechanistically linked to the production of several molecules that promote inflammation. Inhibiting HIF-1 led to a decrease in the production of these inflammatory molecules, indicating that increased activity of HIF-1 causes inflammation in sarcoidosis patients.
It remains unclear what causes this abundance of HIF-1α. It is possible that specific modifications of this factor prevent it from degrading, resulting in higher levels. By identifying a link between HIF-1 and inflammation, these findings open up potential new avenues of the treatment for sarcoidosis patients.




