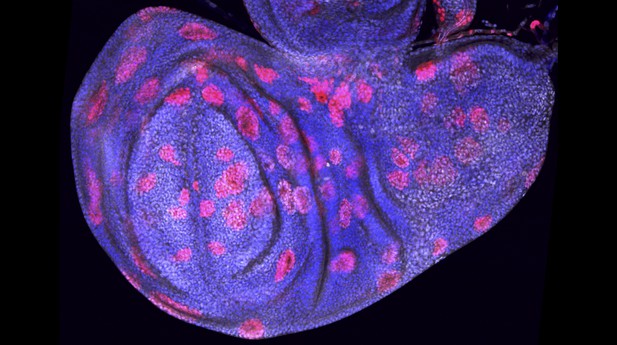
Patches of cells from a fruit fly (red), with the DNA stained (blue). Image credit: Wong et al. (CC BY 4.0)
Cancer arises when cells in the body divide and grow excessively. These cells will often also take up more glucose than normal cells and break it down into another chemical known as lactate faster. This change to the chemical reactions happening within the cell, also called a metabolic change, is required to help produce the extra DNA, proteins and fatty molecules that it needs to grow.
Elevated levels of certain proteins can trigger the changes that lead to the growth of tumors. MYC (called dMyc in fruit flies) is one of these proteins. It controls cell division and increases the production of enzymes that break down glucose. Hipk is another protein that can induce tumor growth in fruit flies, but how it does so was unknown.
Here, Wong et al. show that high levels of Hipk boost glucose metabolism and accumulation of dMyc protein in fruit fly cells. They also describe the link between increased glucose metabolism and uncontrolled cell division.
First, fruit fly cells were fed a fluorescent molecule similar to glucose that cannot be broken down by the cells. This experiment established that glucose uptake increases in cells with too much Hipk. These cells also break down glucose faster, confirming that they have increased glucose metabolism.
Cells with high levels of Hipk also activate the genes that generate the enzymes involved in glucose breakdown, and increase the activity of the gene coding for dMyc. Levels of the dMyc protein are higher in these cells, which was shown by staining the cells with fluorescent molecules that specifically bind the dMyc protein. It is this buildup of dMyc protein that activates the genes coding for the enzymes responsible for glucose breakdown. PFK2 is one of these enzymes.
Finally, Wong et al. inhibited the production of the enzymes that are elevated in cells with high Hipk. Stopping the production of PFK2 prevents both tumor growth and the accumulation of dMyc protein. This shows that high levels of dMyc increase PFK2 levels, leading to further dMyc buildup, and creating a loop that links the uncontrolled cell division caused by too much dMyc and the shift to higher glucose metabolism.
These results highlight new potential targets for cancer therapy, showing that targeting glucose metabolism may reduce, or even stop, tumor growth.




