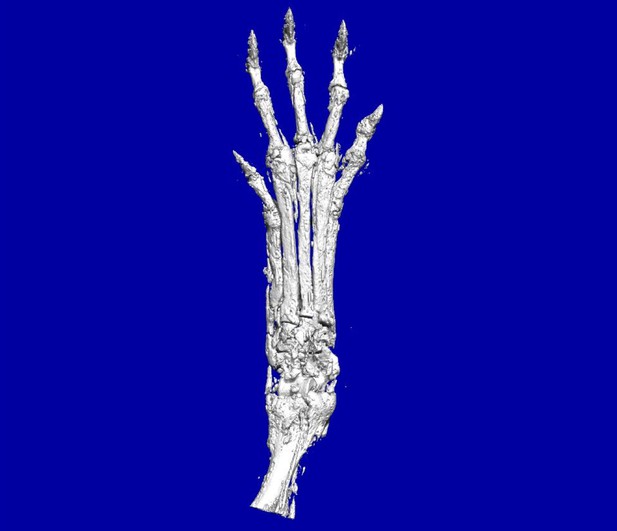
Bones in the hands of mice where Ptch1 has been deleted from mesenchymal cells, showing excess cell growth on bone surfaces and distorted joints. Image credit: Qi Deng (CC BY 4.0)
Bone and cartilage tumors are among the most common tumors in the skeleton, often affecting the limbs. Bone tumors, also called osteosarcomas, usually occur in growing children and teenagers, and they are often resistant to conventional chemo- and radio-therapies. Surgery is the only treatment option, but this can lead to long-lasting damage that impairs the quality of life of these patients. Thus, there is a need to find new drug targets for these diseases. Unfortunately, no good laboratory-based systems exist that mimic these human cancers, hindering research into these tumors.
One way to create a laboratory-based model for cartilage tumors and osteosarcomas is to reproduce the signaling that is present in the human tumors in a mouse. A signaling pathway called Hedgehog signaling is overactive in human cartilage and bone tumors. The activity of this pathway can be increased by deleting a gene called Ptch1; but mice do not form tumors when this gene is deleted in their mature cartilage and bone cells.
Now, Deng, Li et al. report that deleting Ptch1 in mesenchymal stem cells, early-stage cells that can give rise to cartilage and bone cells, generates a mouse model for osteosarcoma and cartilage tumors. The mice with these Ptch1 deficient cells developed tumors with overactive Hedgehog signaling in cartilage and bone. Deng, Li et al. also performed biochemical experiments to show that Hedgehog signaling turned on another signaling pathway called Wnt signaling. Treating the mice that had mesenchymal cells lacking Ptch1 with a drug that inhibits Wnt signaling reduced the growth of cartilage and bone tumors.
These data suggest that deleting Ptch1 in mouse mesenchymal stem cells can mimic human cartilage tumors and osteosarcomas. More experiments will be needed to explain how the Hedgehog and Wnt signaling pathways interact in these tumors. Finally, further studies will need to investigate if inhibiting Wnt signaling might become a useful therapy for human patients with osteosarcoma in the future.




