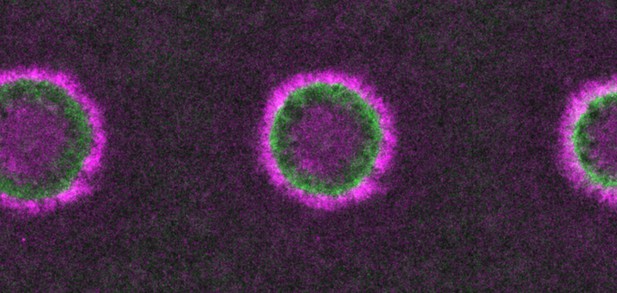
Increased levels of RhoGDI lead to a decrease in Rho (green) and Cdc42 (magenta) activity around wounds in frog cells. Image credit: Golding, Visco et al. (CC BY 4.0)
Organisms rely on many signaling molecules to control how their cells grow, divide and heal. For example, when the cell membrane is damaged, two signaling proteins, Rho and Cdc42, are recruited to wounds and activated to promote repair. Active Rho and active Cdc42 form two concentric rings at the membrane to direct the closure of the wound.
Rho and Cdc42 belong to the RhoGTPase family, a group of proteins that act as molecular switches and alternate between active and inactive forms. At the level of the cell, RhoGTPases are only active in the tiny patches of the membrane where they bind. However, individual proteins hop on and off membranes in a matter of seconds, only staying bound for short periods. This mechanism is controlled by a regulatory protein known as RhoGDI, and it allows RhoGTPases to form precise patterns of activity at membranes – such as the rings that surround a wound site. However, it was not known exactly how RhoGDI regulates the activity of RhoGTPases over space and time, partly because it is difficult to study these proteins in the laboratory.
To fill this knowledge gap, Golding, Visco et al. developed new fluorescent probes to track Rho and Cdc42 in wounded cells from frogs and on artificial membranes. The experiments showed that pools of inactive Cdc42 accumulated on membranes, alongside the active form of the protein. RhoGDI removed both active and inactive RhoGTPases from artificial and frog cell membranes. In fact, removing active Rho and Cdc42 proteins from the cell membrane was necessary to form the spatial patterns of RhoGTPase activity observed in wounded frog cells.
The findings of Golding, Visco et al. help to understand how RhoGDI proteins regulate RhoGTPases and provide new tools to further study these proteins. In humans, mutations in either RhoGDI or Cdc42 are responsible for severe conditions such as Nephrotic Syndrome Type 8 or Takenouchi-Kosaki syndrome. In the future, this work may aid the development of treatments and cures for these conditions.




