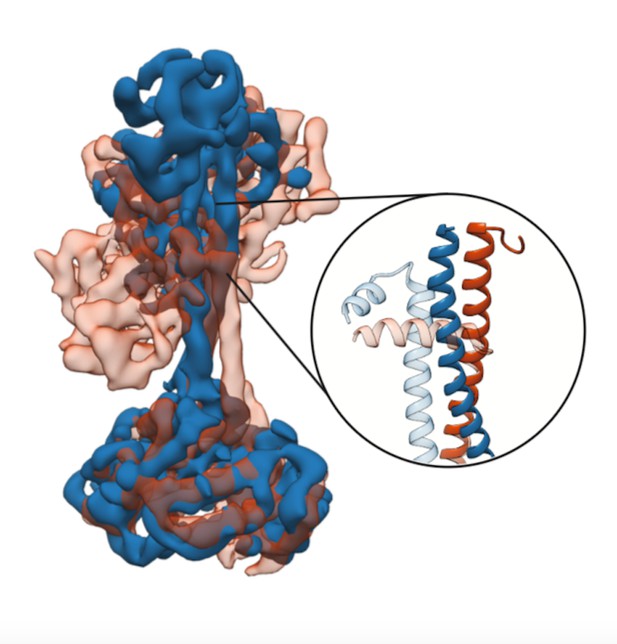
3D structure of soluble guanylate cyclase, showing the β H-NOX domain in the detail. Image credit: Horst, Yokom et al. (CC BY 4.0)
In humans and other animals, as the heart pumps blood around the body, the blood exerts pressure on the walls of the blood vessels, much like water flowing through a hose. Our blood pressure naturally varies over the day, generally increasing when we are active and decreasing when we rest. However, if blood pressure remains high for extended periods of time it can lead to heart attacks, strokes and other serious health conditions.
In 2013, a new drug known as Adempas was approved to treat high blood pressure in the lungs. This drug helps a signaling molecule in the body called nitric oxide to activate an enzyme that widens blood vessels and in turn lower blood pressure. Previous studies have found that the enzyme – called soluble guanylate cyclase (sGC) – contains several distinct domains and that nitric oxide binds to a domain known as β H-NOX. However, it was not clear how β H-NOX and the other three domains fit together to make the three-dimensional structure of the enzyme, or how nitric oxide and Adempas activate it.
To address this question, Horst, Yokom et al. used a technique called cryo-electron microscopy to determine the three-dimensional structures of the inactive and active forms of a soluble guanylate cyclase from a moth known as Manduca sexta. To produce the active form of the enzyme, soluble guanylate cyclase was incubated with both nitric oxide and a molecule called YC-1 that works in similar way to Adempas. The structures revealed that nitric oxide and YC-1 caused β H-NOX and another domain to rotate by 71. This in turn caused the remaining two domains – known as the coiled-coil domains – to change shape, and all of these movements together led to the activated enzyme. The structures also revealed that YC-1 bound to a site on the enzyme between β H-NOX and the coiled-coil domains.
Understanding how a drug for a particular condition works makes it much easier to develop new drugs that are more effective at treating the same condition or are tailored to treat other diseases. Therefore, these findings will allow pharmaceutical companies and other organizations to develop new drugs for high blood pressure and other cardiovascular diseases in a much more precise way.




