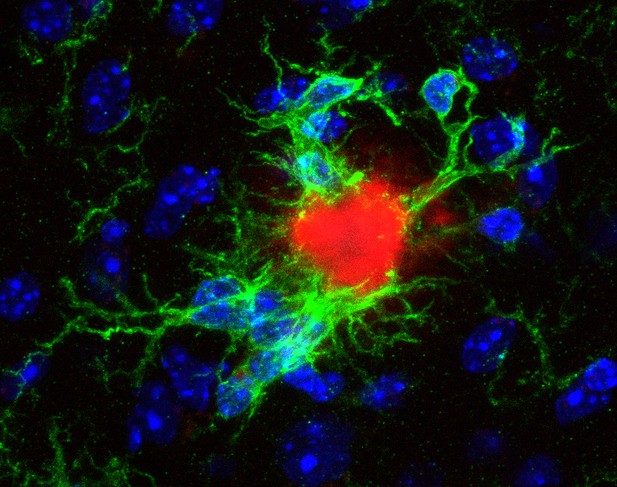
Microglia surrounding a compacted plaque in the brain of a mouse with hallmarks of Alzheimer’s disease. Image credit: Laura Sebastian Monasor (CC BY 4.0)
Alzheimer’s disease is a progressive, irreversible brain disorder. Patients with Alzheimer’s have problems with memory and other mental skills, which lead to more severe cognitive decline and, eventually, premature death. This is due to increasing numbers of nerve cells in the brain dying over time. A distinctive feature of Alzheimer’s is the abnormally high accumulation of a protein called amyloid-β, which forms distinctive clumps in the brain termed ‘plaques’.
The brain has a type of cells called the microglia that identify infections, toxic material and damaged cells, and prevent these from building up by clearing them away. In Alzheimer’s disease, however, the microglia do not work properly, which is thought to contribute to the accumulation of amyloid-β plaques. This means that people with mutations in the genes important for the microglia activity are also at higher risk of developing the disease.
Although problems with the microglia play an important role in Alzheimer’s, researchers still do not fully understand why microglia stop working in the first place. It is also not known exactly when and how the microglia change as Alzheimer’s disease progresses. To unravel this mystery, Sebastian Monasor, Müller et al. carried out a detailed study of the molecular ‘fingerprints’ of microglia at each key stage of Alzheimer’s disease.
The experiments used microglia cells from two different strains of genetically altered mice, both of which develop the hallmarks of Alzheimer’s disease, including amyloid-β plaques, at similar rates. Analysis of the proteins in microglia cells from both strains revealed distinctive, large-scale changes corresponding to successive stages of the disease – reflecting the gradual accumulation of plaques. Obvious defects in microglia function also appeared soon after plaques started to build up.
Microscopy imaging of the brain tissue showed that although amyloid-β plaques appeared at the same time, they looked different in each mouse strain. In one, plaques were more compact, while in the other, plaques appeared ‘fluffier’, like cotton wool. In mice with more compacted plaques, microglia recognized the plaques earlier and stopped working sooner, suggesting that plaque structure and microglia defects could be linked.
These results shed new light on the role of microglia and their changing protein ‘signals’ during the different stages of Alzheimer’s disease. In the future, this information could help identify people at risk for the disease, so that they can be treated as soon as possible, and to design new therapies to make microglia work again.




