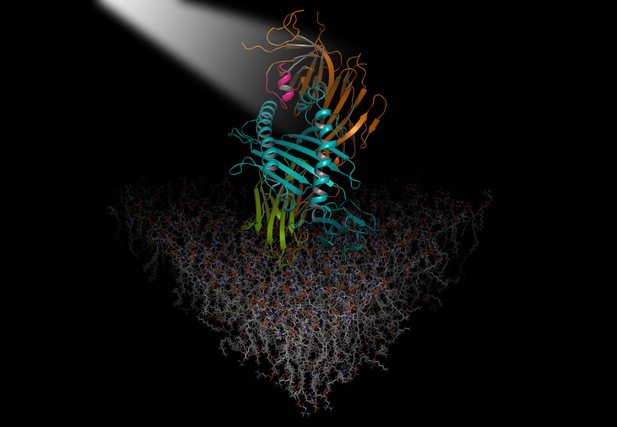
An illuminated "scoop loop" of TAPBPR protein (magenta and orange) in complex with the MHC (teal and green) embedded in the cell membrane. Image credit: Christoph Thomas and Robert Tampé (CC BY 4.0)
Cells in the body keep the immune system informed about their health by showing it fragments of the proteins they have been making. They display these fragments, called peptides, on MHC molecules for passing immune cells to inspect. That way, if a cell becomes infected and starts to make virus proteins, or if it becomes damaged and starts to make abnormal proteins, the immune system can ‘see’ what is happening inside and trigger a response.
MHC molecules each have a groove that can hold one peptide for inspection. For the surveillance system to work, the cell needs to load a peptide into each groove before the MHC molecules reach the cell surface. Once the MHC molecules are on the cell surface, the peptides need to stay put; if they fall out, the immune system will not be able to detect them. The problem for the cell is that not all peptides fit tightly into the groove, so the cell needs to check each one before it goes out. It does this using a protein called TAPBPR.
TAPBPR has a finger-like structural feature called the "scoop loop", which fits into the end of the MHC groove while the molecule waits for a peptide. It was not clear, however, what this loop actually does. To investigate, Sagert et al. mutated the scoop loop of TAPBPR to see what happened to MHC loading in test tubes.
The experiments revealed that the scoop loop plays two important roles. The first is to keep the MHC molecule stable when it is empty, and the second is to hinder unsuitable peptides from binding. The scoop loop sticks into one side of the groove like a tiny hairpin, so that pushed-out, poorly fitting peptides cannot reattach. At the same time, it holds the MHC molecule steady until a better peptide comes along and only releases when the new peptide has slotted tightly into the groove.
Understanding how cells choose which peptides to show to the immune system is important for many diseases. If cells are unable to find a suitable peptide for a particular illness, it can stop the immune system from mounting a strong response. Further research into this quality control process could aid the design of new therapies for infectious diseases, autoimmune disorders and cancer.




