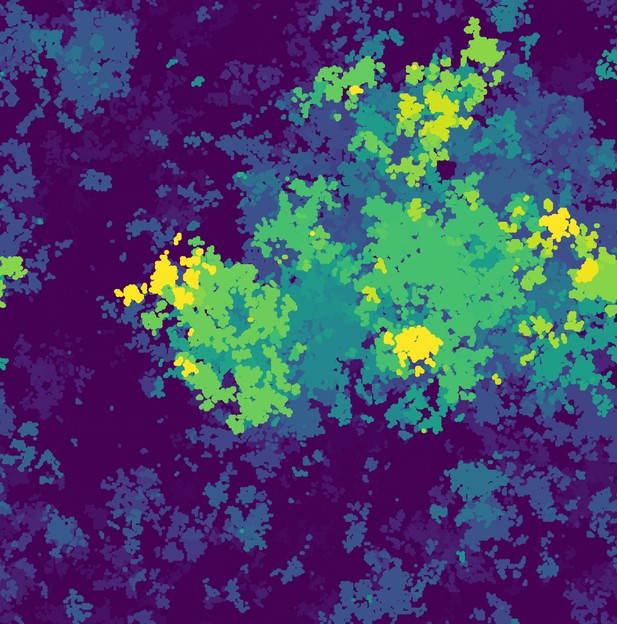
Image showing a ‘gene-sharing’ community of bacteria (shown in yellow) overgrowing other bacteria (shown in blue) that do not engage in horizontal gene transfer. Image credit: Bram van Dijk (CC BY 4.0)
Most animals, including humans, inherit genes from their parents. However, bacteria and other microorganisms can also acquire genes from members of the same generation. This process, called horizontal gene transfer (HGT for short), allows bacteria to quickly adapt to new environments. For example, rather than waiting for rare mutations to arise, bacteria can pick up ‘tried and true’ genes from their neighbours which allow them to exploit new resources or become resistant to antibiotics.
But gene sharing comes at a cost. For instance, taking up DNA is an energetically costly process and exposes bacteria to so-called selfish genes which replicate at the expense of other more useful genes in the genome. Given the costs and the threat of selfish genes, it remained unclear whether HGT is still beneficial in a stable environment where no new resources or antibiotics are present.
Here, van Dijk et al. used mathematical modelling to examine how gene sharing affects the growth rate of bacterial colonies living in a stable environment. The experiments showed that bacteria are able to take up new sequences of DNA even in the presence of selfish genes. This allows communities of bacteria to retain genes that provide a small benefit that would otherwise be lost from the population, even when taking up DNA imposes a cost upon the individual. van Dijk et al. found that this collective behaviour cannot evolve in well-mixed bacterial populations, but readily emerged in more structured populations, such as biofilms.
This work demonstrates how HGT, a key component of bacterial evolution, has allowed bacteria to coexist with harmful selfish genes. It also provides insights into how genes persist and spread through bacterial communities, which has implications for our understanding of antibiotic resistance.




