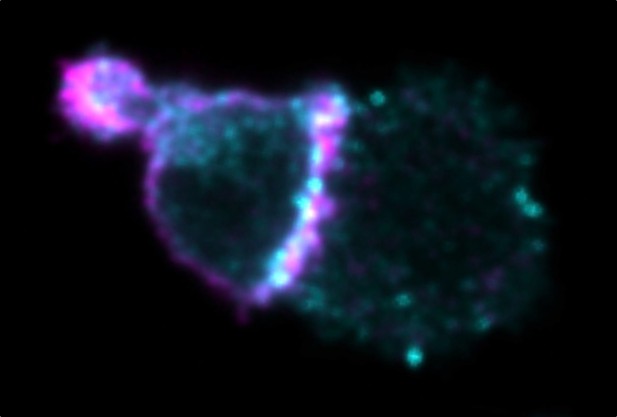
Human natural killer cell (left) joined to a target cancer cell (right) with phosphorylated Pyk2 in cyan and CD56 in magenta. Image credit: Gunesch et al. (CC BY 4.0)
The immune system deploys different cell types to take out cancer cells. True to their name, one type of immune cell known as natural killer cells kills tumor target cells by releasing toxic proteins that kill the harmful cells. In humans, these immune cells are defined, among other things, by the presence of a protein called CD56 on their cell surface. This protein (which is also known as NCAM) is thought to help cells to stick to their surroundings and control their movements. However, it was not clear whether CD56 also plays a role in the destructive abilities of natural killer cells.
Gunesch et al. have now looked to see what would happen if natural killer cells lacked CD56 on their surface. The experiments included deleting the gene for CD56 from two kinds of human natural killer cell that are commonly grown in the laboratory (called NK92 and YTS). In both cases, the cells lacking CD56 killed fewer cancer cells than the unedited natural killer cells. The NK92 cells were much more affected by the loss of CD56 than the YTS cells, and after Gunesch et al. compared the two kinds of cell they identified another protein called Pyk2 as the potential reason behind the difference.
The Pyk2 protein is known to help a natural killer cell latch onto target cancer cells and release its toxic proteins. To do this, Pyk2 must first be activated with phosphate groups via a process known as phosphorylation. Gunesch et al. showed that Pyk2 protein in unedited NK92 cells was more highly phosphorylated than those of the YTS cells, and that Pyk2 activation by phosphorylation was greatly decreased in NK92 cells when the gene for CD56 was deleted. Together these and other results suggest that CD56 on natural killer cells helps to promote Pyk2 to activate the cells’ cancer-killing abilities through Pyk2 phosphorylation, especially in NK92 cells.
These findings open up new lines of investigation into the relationship between sticky surface proteins and the activation of immune cells. They may also have important implications for the use of the immune system to treat cancer via immunotherapy.




