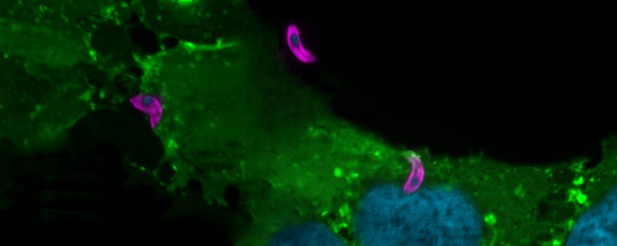
Three T. gondii parasites containing non-myristoylated MIC7 protein (pink) trying to invade host cells (shown with green membranes). Image credit: Luis Vigetti (CC BY 4.0)
A microscopic parasite known as Toxoplasma gondii infects around 30% of the human population. Most infections remain asymptomatic, but in people with a compromised immune system, developing fetuses and people infected with particular virulent strains of the parasite, infection can be fatal. T. gondii is closely related to other parasites that also infect humans, including the one that causes malaria. These parasites have complex lifecycles that involve successive rounds of invading the cells of their hosts, growing and then exiting these cells. Signaling proteins found at specific locations within parasite cells regulate the ability of the parasites to interact with and invade host cells. Sometimes these signaling proteins are attached to membranes using lipid anchors, for example through a molecule called myristic acid.
An enzyme called NMT can attach myristic acid to one end of its target proteins. The myristic acid tag can influence the ability of target proteins to bind to other proteins, or to membranes. Previous studies have found that drugs that inhibit the NMT enzyme prevent the malaria parasite from successfully invading and growing inside host cells. The NMT enzyme from T. gondii is very similar to that of the malaria parasite. Broncel et al. have shown that the drug developed against P. falciparum also inhibits the ability of T. gondii to grow. These findings suggest that drugs against the NMT enzyme may be useful to treat diseases caused by T. gondii and other closely-related parasites.
Broncel et al. also identified 65 proteins in T. gondii that contain a myristic acid tag using an approach called proteomics. One of the unexpected ‘myristoylated’ proteins identified in the experiments is known as MIC7. This protein was found to be transported onto the surface of T. gondii parasites and is required in its myristoylated form for the parasite to successfully invade host cells. This was surprising as myristoylated proteins are generally thought to not enter the pathway that brings proteins to the outside of cell.
These findings suggest that myristic acid on proteins that are secreted can facilitate interactions between cells, maybe by inserting the myristic acid into the cell membrane.




