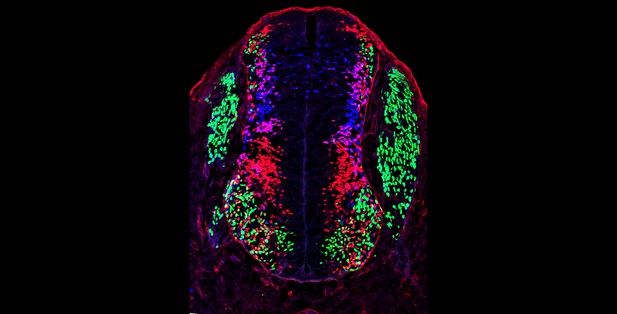
Cross section of the spinal cord of a mouse; the colors mark different types of neurons. Image credit: Chang et al. (CC BY 4.0)
The spinal cord is an information superhighway that connects the body with the brain. There, circuits of neurons process information from the brain before sending commands to muscles to generate movement. Each spinal cord circuit contains many types of neurons, whose identity is defined by the set of genes that are active or ‘expressed’ in each cell.
When a gene is turned on, its DNA sequence is copied to produce a messenger RNA (mRNA), a type of molecule that the cell then uses as a template to produce a protein. MicroRNAs (or miRNAs), on the other hand, are tiny RNA molecules that help to regulate gene expression by binding to and ‘deactivating’ specific mRNAs, stopping them from being used to make proteins.
Mammalian cells contain thousands of types of microRNAs, many of which have unknown roles: this includes MiR34/449, a group of six microRNAs found mainly within the nervous system. By using genetic technology to delete this family from the mouse genome, Chang et al. now show that MiR34/449 has a key role in regulating spinal cord circuits.
The first clue came from discovering that mice without the MiR34/449 family had unusual posture and a tendency to walk on tiptoe. The animals were also more sensitive to heat, flicking their tails away from a heat source more readily than control mice.
At a finer level, the spinal cords of the mutants contained greater numbers of cells in which two genes, Satb1 and Satb2, were turned on. Compared to their counterparts in control mice, the Satb1/2-positive neurons also showed differences in the rest of the genes they expressed. In essence, these neurons had a different genetic profile in MiR34/449 mutant mice, therefore disrupting the neural circuit they belong to.
Based on these findings, Chang et al. propose that in wild-type mice, the MiR34/449 family fine-tunes the expression of Satb1/2 in the spinal cord during development. In doing so, it regulates the formation of the spinal cord circuits that help to control movement. More generally, these results provide clues about how miRNAs help to determine cell identities; further studies could then examine whether other miRNAs contribute to the development and maintenance of neuronal circuits.




