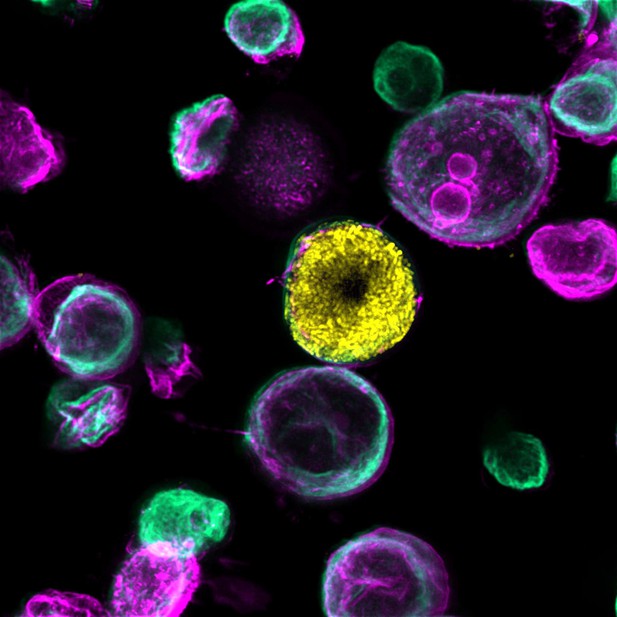
Microscopy image of a bladder-chip showing a uroepithelial cell (stained green) that has been infected with bacteria that have grown inside the cell to form a community (shown in yellow). Image credit: Kunal Sharma (CC BY 4.0)
Urinary tract infections are one of the most common reasons people need antibiotics. These bacterial infections are typically caused by uropathogenic Escherichia coli (also known as UPEC), which either float freely in the urine and wash away when the bladder empties, or form communities inside cells that the bladder struggles to clear. It is possible that the bacteria living within cells are also more protected from the immune system and antibiotics. But this is hard to study in animal models.
To overcome this, Sharma et al. built a ‘bladder-chip’ which mimics the interface between the blood vessels and the tissue layers of the human bladder. Similar chip devices have also been made for other organs. However, until now, no such model had been developed for the bladder.
On the chip created by Sharma et al. is a layer of bladder cells which sit at the bottom of a channel filled with diluted human urine. These cells were infected with UPEC, and then imaged over time to see how the bacteria moved, interacted with the bladder cells, and aggregated together. Immune cells from human blood were then added to a vascular channel underneath the bladder tissue, which is coated with endothelial cells that normally line blood vessels. The immune cells rapidly crossed the endothelial barrier and entered the bladder tissue, and swarmed around sites of infection. In some instances, they released the contents of their cells to form net-like traps to catch the bacteria. But these traps failed to remove the bacteria living inside bladder cells.
Antibiotics were then added to the urine flowing over the bladder cells as well as the vascular channel, similar to how drugs would be delivered in live human tissue. Sharma et al. discovered that the antibiotics killed bacteria residing in bladder cells slower than bacteria floating freely in the urine. Furthermore, they found that bacteria living in tightly packed communities within bladder cells were more likely to survive treatment and go on to re-infect other parts of the tissue.
Antibiotic resistance is a pressing global challenge, and recurrent urinary tract infections are a significant contributor. The bladder-chip presented here could further our understanding of how these bacterial infections develop in vivo and how good antibiotics are at removing them. This could help researchers identify the best dosing and treatment strategies, as well as provide a platform for rapidly testing new antibiotic drugs and other therapies.




