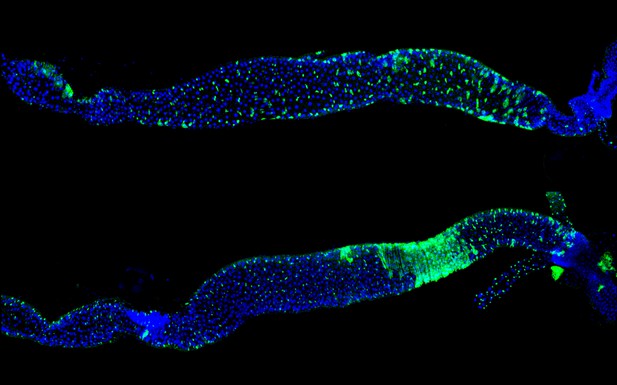
Fruit flies lacking vinculin (bottom) have a faster rate of cell turn over (green) in their guts compared to their normal counterparts (top). Image credit: Jérôme Bohère
Mechanical changes in the environment have recently emerged as important signals of cell division and production of specialised cell types. Exactly how these forces are sensed and contribute to this process in living tissues, however, remains unclear.
This question is particularly relevant in the lining of the gut. Endlessly exposed to intense mechanical stress and the passage of food, this tissue must constantly heal and renew itself. The intestinal cells that absorb nutrients from food, for example, are continually replaced as older cells are lost. This is made possible by immature ‘progenitor’ cells in the intestine dividing and maturing into various specialised cells – including fully functional absorptive cells – upon receiving the right mechanical and chemical signals. Errors in this carefully regulated process can result in too many or too few cells of the correct kind being produced, potentially leading to disease.
To explore how mechanical forces may help to control the renewal and maturation of new intestinal cells, Bohère et al. examined the role of vinculin in the guts of fruit flies (where cell fate decisions involve mechanisms largely similar to humans). Vinculin can regulate cell fate, sense mechanical forces, and interact with the complex structures that physically connect cells to each other.
Genetically altered flies that lacked vinculin had enlarged guts containing many more absorptive cells than those of normal flies, suggesting that the vinculin protein prevents over-production of these cells. Further experiments revealed that vinculin worked exclusively in the precursors of absorptive cells, keeping them in an immature state until new mature absorptive cells were required. This was achieved by vinculin acting upon – and potentially strengthening – the junctions connecting cells together. Finally, increasing the force within cells was shown to facilitate vinculin recruitment to these junctions.
This study clarifies the role that mechanical forces at the interface between cells play in controlling when and how intestinal progenitors mature in an organism. If these findings are confirmed in mammals, Bohère et al. hope that they could inform how tissues cope with the changing mechanical landscape associated with ageing and inflammation.




