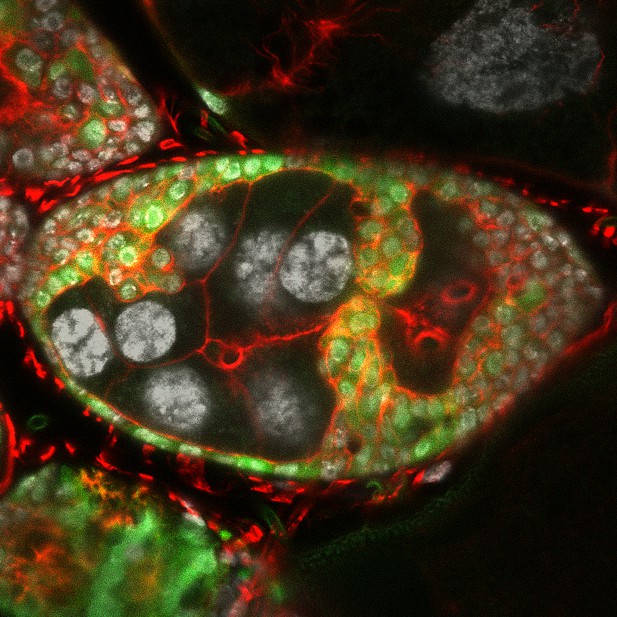
Cross-section of structures known as the egg chambers that make up a fruit-fly ovary. When combined with enhanced Keap1-Nrf2 oxidative stress pathway activation (by means of turning on the gene Keap1), loss of apical-basal polarity in the epithelial cells (labeled in green) caused them to form invasive in-roads into the neighboring germline-cell compartment (the cells in the center of the image with large nuclei labeled in white). The protein actin (labeled in red) is enriched in the invading group of cells. Image credit: Deeptiman N Chatterjee (CC BY 4.0)
In the body, most cells exhibit some form of spatial asymmetry: the compartments within the cell are not evenly distributed, thereby allowing the cells to know whether a surface is on the ‘outside’ or the ‘inside’ of a tissue or organ. In the cells of epithelial tissues, which line most of the cavities and the organs in the body, this asymmetry is known as apical-basal polarity. Maintaining apical-basal polarity in epithelial cells is one of the main barriers that stops cancer cells from invading other tissues, which is the first step of metastasis, the process through which cancer cells leave their tissue of our origin and spread to distant locations in the body.
In the fruit fly Drosophila melanogaster, scientists have engineered cells in several tissues to stop producing the proteins that help establish apical-basal polarity, in an effort to study the earliest steps of tumor formation. Unfortunately, these experiments frequently lead to rampant metastasis, making it difficult to identify the earliest changes that make the tumor cells more likely to become invasive. Therefore, finding a tissue in which loss of apical-basal polarity does not cause aggressive cancer progression is necessary to address this gap in knowledge.
The epithelial cell layer lining the ovaries of fruit flies may be such a tissue. When these cells lose their apical-basal polarity, rather than becoming metastatic and spreading to distant organs, they interleave with each other, forming a tumorous growth that only invades into the neighboring compartment. Chatterjee et al. used this system to study individual invasive cells. They wanted to know whether the genes that these cells switch on and off are known to be involved in human cancers, and if so, which of them control the invasive behavior of tumor cells.
Chatterjee et al. determined that when cells in the fruit-fly ovary lost their polarity, they turned genes on and off in a pattern similar to that seen both in mammalian cancers and in tumors from other fly tissues. One of the notable changes they observed in the ovarian cells that lost apical-basal polarity was the activation of the Keap1/Nrf2 oxidative-stress signaling pathway, which normally protects cells from damage caused by excessive oxidation. In the ovarian cells, however, the activation of these genes also led to aggressive invasion of the collective tumor cells into the neighboring compartment.
Interestingly, this increase in invasiveness was characterized by polarized changes within the cells, specifically in the scaffolding that allows cells to keep their shape and move: the edge of the cells leading the invasion had greater levels of a protein called actin, which enables the cells to protrude into the neighboring compartments.
Chatterjee et al. have identified a new mechanism that impacts the migratory behavior of cells. Insights from their findings will pave the way for a better understanding of how and when this mechanism plays a role in metastasis.




