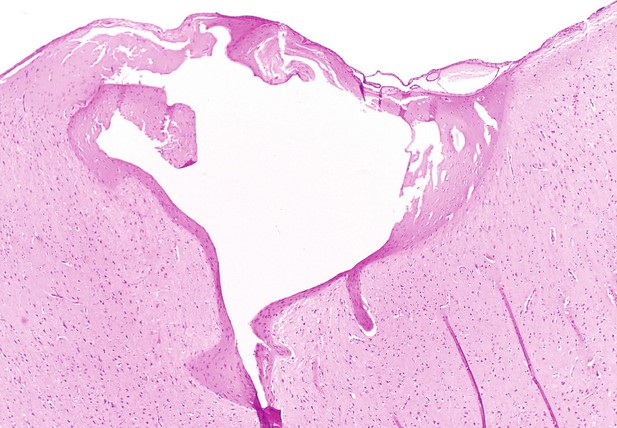
Tissue extracted from the brain of a sheep which has been lesioned (white hole) with the microelectrode array created by Bray, Clarke et al. Image credit: Brain Interfacing Laboratory (CC BY 4.0)
Over the past three decades, the field of neuroscience has made significant leaps in understanding how the brain works. This is largely thanks to microelectrode arrays, devices which are surgically implanted into the outermost layer of the brain known as the cortex. Once inserted, these devices can precisely monitor the electrical activity of a few hundred neurons while also stimulating neurons to reversibly modulate their activity.
However, current microelectrode arrays are missing a key function: they cannot irreversibly inactivate neurons over long-time scales. This ability would allow researchers to understand how networks of neurons adapt and re-organize after injury or during neurodegenerative diseases where brain cells are progressively lost.
To address this limitation, Bray, Clarke, et al. developed a device capable of creating consistent amounts of neuron loss, while retaining the crucial ability to record electrical activity following a lesion. Calibration tests in sheep and pigs provided the necessary parameters for this custom circuit, which was then verified as safe in non-human primates. These experiments demonstrated that the device could effectively cause neuron loss without compromising the recording capabilities of the microelectrode array.
By seamlessly integrating neuron inactivation with monitoring of neuronal activity, scientists can now investigate the direct effects of such damage and subsequent neural reorganization. This device could help neuroscientists to explore neural repair and rehabilitation after brain cell loss, which may lead to better treatments for neurodegenerative diseases. In addition, this technique could offer insights into the interactions between neural circuits that drive behavior, enhancing our understanding of the complex mechanisms underlying how the brain works.




