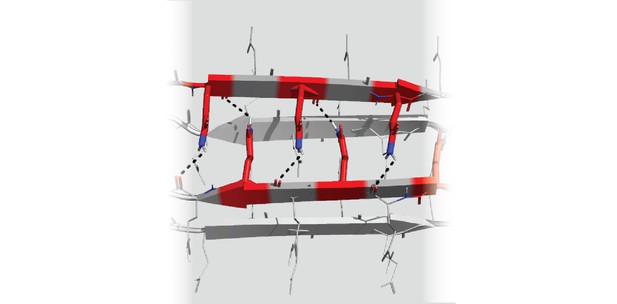
Structure of the polyglutamine protein that will initiate the formation of the amyloid that causes Huntington’s and other related neurodegenerative diseases. Image credit: Celeste Sagui and Randal Halfmann (CC BY 4.0)
Diseases that typically occur later in life, such as Alzheimer’s, are often caused by specific proteins clumping together into structures known as amyloids. Once the process starts, amyloids will continue to form, leading to worse symptoms that cannot be cured. The best way to treat these diseases is therefore to stop amyloids from arising in the first place.
Amyloids initially develop by proteins coming together to create an unstable structure referred to as the nucleus. The instability of the nucleus means it cannot be observed directly, making it hard to study this nucleation process. To overcome this, Kandola, Venkatesan et al. investigated the simplest protein known to form an amyloid – polyglutamine, which is made up of a chain of repeating building blocks known as amino acids.
Polyglutamine forms only one type of amyloid which is associated with nine neurodegenerative diseases, including Huntington’s disease. However, it only does this when its chain of amino acids exceeds a certain length, suggesting that a specific structure may be required for nucleation to begin.
Kandola, Venkatesan et al. made alternative versions of the polyglutamine protein which each contained slightly different sequences of amino acids that will alter the way the protein folds. They then tested how well these different variants could form amyloids in yeast cells. This revealed that in order to join together into a nucleus, polyglutamine needs to be able to fold into a zipper shape made up of four interlocking strands. The length of the protein required to form this shape is also the same length that causes the amyloid associated with neurodegenerative diseases.
Kandola, Venkatesan et al. also found that polyglutamine tends to bind to nuclei that have already formed in a way that hinders their growth. This ‘self-poisoning’ affect could potentially be exploited as a way to pre-emptively stop amyloids from initially arising.
These findings have uncovered a potential therapeutic strategy for blocking amyloid formation that could eventually benefit people with or at risk of developing neurodegenerative diseases linked to polyglutamine. Additionally, this approach provides a blueprint for understanding how other proteins undergo amyloid nucleation, including those responsible for Alzheimer’s, Parkinson’s, and other diseases.




