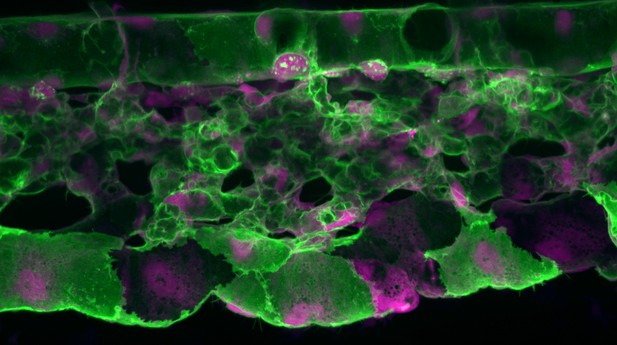
Microscopy image of the vascular system in the tail region of a zebrafish embryo. Plasma membranes are shown in green, with cytoplasm and nuclei in red. Precursors of hematopoietic stem cells emerge from the aorta (vessel at the top of the image). The other vessels are venous components, with the middle part organized as a network for newly born hematopoietic stem cells and progenitors to home to. Image credit: Anne Schmidt (CC BY 4.0).
In mammals and other animals with backbones, the cells that will make up blood and immune cells are generated during a very narrow timeframe in embryonic development. These cells, called hematopoietic stem cells and progenitors (or HSPCs for short), emerge from tissue known as hemogenic endothelium that makes up the floor of early blood vessels.
For HPSCs to eventually specialise into different types of blood and immune cells, they require diverse migratory and homing properties that, ultimately, will determine the specific type of functions they exert. An important question for scientists studying the development of different blood and immune cell types is when this commitment to functional diversity is established. It could, for example, arise due to cells in the hemogenic endothelium having different origins. Alternatively, the signals that generate hemogenic endothelium cells could be responsible. It is also possible that both explanations are true, and that having different mechanisms involved ensures diversity in populations of HSPCs.
To investigate differences between the HSPCs emerging from the hemogenic endothelium, Torcq et al. studied zebrafish embryos that had been modified so that one of the proteins involved in sensing cell polarity – where the top and bottom of the cell are located – was fluorescent. Live imaging of the embryos showed that two types of cells, with striking differences in morphology, emerge from the hemogenic tissue. In addition, one cell type displays the same polarity as the other vessel cells, whereas the other does not. Torcq et al. also present evidence suggesting that the signals responsible for controlling this cell polarity are provided by surrounding blood vessel cells, supporting the idea of an interplay between the different cell types.
The finding that two different cell types emerge from the hemogenic endothelium, reveals a potential new source of diversity in HSPCs. Ultimately, this is expected to contribute to their functional complexity, resulting in both long-term stem cells that retain their full regenerative potential into adulthood and more specialized blood and immune cells.




