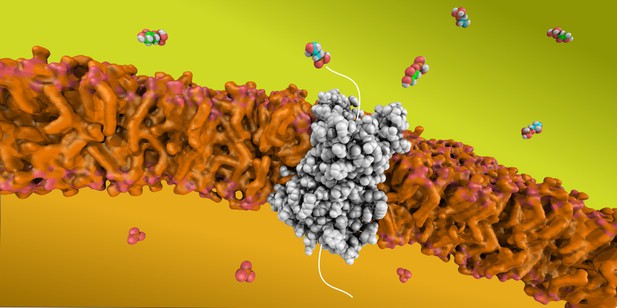
The transport protein GIC-2 (shown in grey) carries the intermediate molecules (shown in green and blue) produced by the first half of glycolysis across the mitochondrial membrane (shown in orange). Image credit: Edmund Kunji (CC BY 4.0)
All living organisms breakdown food molecules to generate energy for processes, such as growing, reproducing and movement. The series of chemical reactions that breakdown sugars into smaller molecules – known as glycolysis – is so important that it occurs in all life forms, from bacteria to humans.
In higher organisms, such as fungi and animals, these reactions take place in the cytosol, the space surrounding the cell’s various compartments. A transport protein then shuttles the end-product of glycolysis – pyruvate – into specialised compartments, known as the mitochondria, where most energy is produced.
However, recently it was discovered that a group of living organisms, called the stramenopiles, have a branched glycolysis in which the enzymes involved in the second half of this process are located in both the cytosol and mitochondrial matrix. But it was not known how the intermediate molecules produced after the first half of glycolysis enter the mitochondria.
To answer this question, Pyrihová et al. searched for transport protein(s) that could link the two halves of the glycolysis pathway. Computational analyses, comparing the genetic sequences of many transport proteins from several different species, revealed a new group found only in stramenopiles. Pyrihová et al. then used microscopy to visualise these new transport proteins – called GIC-1 and GIC-2 – in the parasite Blastocystis, which infects the human gut, and observed that they localise to mitochondria.
Further biochemical experiments showed that GIC-1 and GIC-2 can physically bind these intermediate molecules, but only GIC-2 can transport them across membranes. Taken together, these observations suggest that GIC-2 links the two halves of glycolysis in Blastocystis.
Further analyses could reveal corresponding transport proteins in other stramenopiles, many of which have devastating effects on agriculture, such as Phytophthora, which causes potato blight, or Saprolegnia, which causes skin infections in farmed salmon. Since human cells do not have equivalent transporters, they could be new drug targets not only for Blastocystis, but for these harmful pathogens as well.




