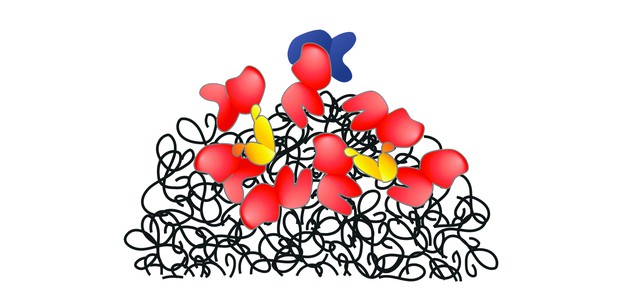
Hsp70 (red) is recruited to harmful aggregates of misfolded proteins (black) with help of Hsp110 (blue) and other co-chaperones (yellow). Image credit: Adapted from Sztangierska et al. (CCBY 4.0).
For proteins to accurately carry out their role in the cell, they must first be precisely folded into specific 3D shapes. Stress, aging or disease can interfere with this delicate process, leading to misfolded proteins clumping together and causing damage. In response, the cell can deploy ‘chaperones’ which disentangle these aggregates and ensure that proteins recover their proper structure. Chaperones from the Hsp70 protein family, for example, are crucial for cell survival, especially under biologically stressful conditions. Yet Hsp70 proteins cannot perform their role without the assistance of co-chaperones such as Hsp110; why this is the case, however, has remained unclear.
To investigate this question, Sztangierska et al. used a variety of biochemical assays to test how purified human and yeast Hsp70, Hsp110 and other co-chaperones could bind aggregates and recover misfolded proteins. The role of each protein was examined at every stage of the disaggregation process – from the initial aggregate binding, through chaperone-driven changes in aggregate structure to the final protein folding.
The experiments revealed that Hsp110 helps draw Hsp70 to the aggregate surface, breaking down the protein ‘clump’ into smaller pieces which are more easily processed by other chaperones. The results also showed that the various co-chaperones compete for Hsp70 binding; too much of one might interfere with another, emphasizing the need for balance between chaperones for optimal disaggregation.
Overall, these results clarify the role of Hsp110 in the Hsp70 system and reveal several mechanistic details of the protein rescue process. Further experiments will be needed to fully understand these dynamics and identify how they may be relevant to conditions in which harmful protein aggregates are observed, such as Parkinson’s or Alzheimer’s disease.




